Several strategies and software applications help pharmaceutical companies integrate their manufacturing execution systems and enterprise resource planning systems.

Several strategies and software applications help pharmaceutical companies integrate their manufacturing execution systems and enterprise resource planning systems.

Pharmaceutical companies want software vendors to make integrating manufacturing execution systems and enterprise resource planning systems easier.

Industry needs a standard to connect systems and equipment sooner rather than later.
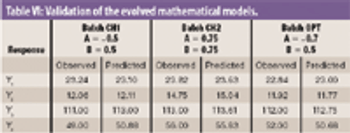
The authors studied the effect of the combination of binders on the flow and compressibility characteristics of the agglomerates of binary combination of lactose and dibasic calcium phosphate dihydrate.

In the wake of economic growth, healthcare reforms, and large-scale industry investment, Turkish pharmaceutical companies are charting their own destiny.
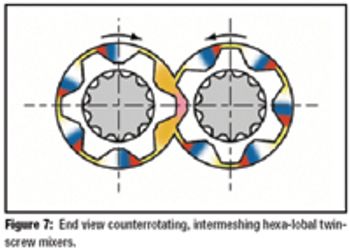
The author describes the benefits, processes, and practicality of using hot-melt extrusion to mix active pharmaceutical ingredients with pharmaceutical-grade polymers.
A recent report shows a decline in NASs and a rise in NME applications in 2007.

Planning ahead will ensure successful efforts to improve packaging and packaging operations.

Advancements add yet another challenge for industry's already overextended regulatory body.

Brief pharmaceutical news items for October 2008.

This article summarizes the classification systems for topical liquid and semisolid dosage forms used for dermatological application and notes some differences between FDA and USP classification.

To err may be human, but to really mess things up, you need management.

Emerging pharmaceutical companies represent an important client base for CROs and CMOs. Lessons learned for successful customer–supplier relations.

Manufacturers seek real-world benefits from investment in QbD and risk-management strategies. This article contains bonus online-exclusive material.

Although the European Union's approval rates of biosimilars for market is increasing slower than expected, its approach may provide an example for other foreign markets.
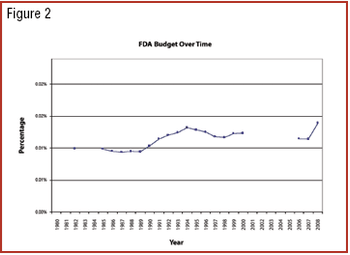
Can previous trends of Democratic and Republican administrations predict industry's future?

A book offers a detailed introduction to the new area of risk evaluation and mitigation strategies.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Cycle design and robustness testing using advanced process analytical technology.

As offshore savings decline, pharmaceutical companies still have a lot of work to do to reduce costs.