
CordenPharma announces expansion of lipid excipients supply for coronavirus vaccine scale up.

CordenPharma announces expansion of lipid excipients supply for coronavirus vaccine scale up.

The investment will give the company the opportunity to accelerate the development of proprietary boron-10 target drugs while it develops its neutron beam accelerator technology for Boron Neutron Capture Therapy.

The company worked with Eurofins Lancaster Laboratories to conduct independent extractables testing that involved eight solvents tested over a 21-day exposure time frame.

Agreements with the PolyPeptide Group and AGC Biologics will scale up production of the Novavax Matrix-M adjuvant.

The VIA Capsule from Cytiva is a liquid nitrogen-free cryogenic shipment system designed to transport cell therapies.

A new clinical and commercial partnership expands Experic’s presence while providing PCI with advanced powder-filling equipment.

The new vaccine would be made with a Newcastle disease virus vector that expresses the immunogenic spike protein of SARS-CoV-2

The center will offer expertise in fill-finish optimization and analytical testing.

The company is voluntarily recalling all lots of Metformin Hydrochloride Extended Release Tablets, USP, 500 mg and 750 mg, because of the detection of N-Nitrosodimethylamine.

The agency is advising healthcare professionals to closely monitor patients with COVID-19 who are receiving chloroquine or hydroxychloroquine for potential side effects.

The agency sent a warning letter to Chloroquineonline.com for selling unapproved products for the treatment of COVID-19.

The Gavi advance market commitment for COVID-19 vaccines is a new financing instrument aimed at incentivizing vaccine manufacturers to produce sufficient and affordable quantities of COVID-19 vaccines.

The quality control from ZeptoMetrix is formulated with purified intact viral particles that have been chemically modified to render them non-infectious and refrigerator stable.

The extension of the company’s product offerings to include the anti-certolizumab pegol antibodies offers critical reagents for the development of assays for TNF alpha inhibitor biologics and their biosimilars.

The kit consists of a test volume of 10 µL, a range of 0.39–25 ng/mL, a lower limit of detection of 0.03 ng/mL, and average inter- and intra-assay coefficients of variation less than 10%.

The company has expanded its microscopy services with the addition of infrared microspectroscopy and hot stage optical microscopy.
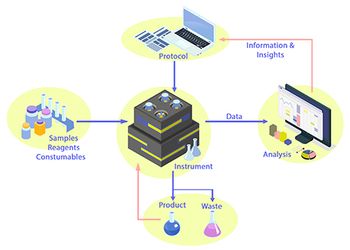
Laboratory automation can reduce the need for in-lab user presence but requires efficient and dependable user-system interface.

Discovery of carcinogenic nitrosamines in three of the world’s most widely prescribed drugs is driving efforts to better detect, control, and prevent their generation in APIs and finished drug products.

The program will invest up to $500 million in biotechnology companies to provide funding and access to Pfizer’s scientific capabilities to continue the biotechnology companies’ clinical development programs.

The medicine is the first outcome of the collaboration between Lilly and AbCellera to create antibody therapies for the prevention and treatment of COVID-19.

FDA and the US Congress support innovation and access to cheaper medicines.

Achieving herd immunity will require testing, data, a vaccine, and public support.

The global COVID-19 pandemic has highlighted the need for the pharmaceutical industry to strengthen its supply chain.

Pharma’s reputation is being boosted in light of the current COVID-19 pandemic efforts.


Despite the clear danger of COVID-19 to global health, vaccine opponents have gained ground, as fearful populations lose faith in the capabilities of industry and government to protect public health.

The companies have entered into a manufacturing agreement for the fill finish supply of lenzilumab for the potential treatment of COVID-19.

The acquisition gives Novovax an annual operating capacity of more that one billion doses of COVID-19 vaccine antigen.

The company recently launched its OmniTop Sample Tubes adjustable volume sampling system, a single-use system that allows for exact sampling.

The new filters extend column life and reduce contamination while simplifying sample preparation.