
The dismissal of all 17 members of the Advisory Committee on Immunization Practices has far-reaching impacts for the industry and beyond.

The dismissal of all 17 members of the Advisory Committee on Immunization Practices has far-reaching impacts for the industry and beyond.

The partnership leverages the Hesperos organ-on-a-chip platform in the preclinical development of Psilera’s lead compound targeting the progressive neurological disorder for which treatment options are few.

Most of the company’s new additions, if not already online, will be scheduled for commissioning, validation, or qualification before the fourth quarter of 2025.

Smart technologies, such as digital and laser printing, incorporated into manufacturing equipment are helping manufacturers align with sustainability goals, according to Sheikh Akbar Ali, general manager and head of Development and Technology for ACG Packaging Materials.

The company describes the compact system as ergonomically designed, providing precision dosing and production floor flexibility.

ICH Q6B provides expectations and a clear framework for the structural characterization of biopharmaceutical products.
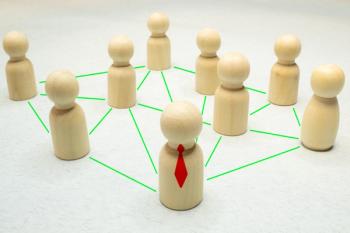
Trends in certain forms of drug delivery, as well as the emergence of artificial intelligence, are playing roles in evolving the nature of partnerships, but there are new types of partnerships gaining steam as well.
Jon Ellis and Simon Vanstone, PhD, go behind the headlines to explore the latest M&A activity and what tariffs and funding changes could mean for mRNA and beyond.

The new lab will be used to develop and scale innovations for the production of medicines and to help secure the supply chain.

EMA has published recommendations to address potential radiopharmaceutical shortages.

The grant encompasses $100,000 each from the Life Sciences Entrepreneurship Center and Berkeley SkyDeck, with SkyDeck having the option to invest an additional $100,000.

As a result of the decision, Shilpa will not be eligible to receive FDA approval for its lenvatinib mesylate generic until February 2036.

A Phase III trial demonstrated mNEXSPIKE’s non-inferior efficacy compared with Moderna’s original COVID vaccine, Spikevax.

Teva’s investigational monoclonal antibody, TEV-53408, has been designed for use in adults with celiac disease, for which a strict and lifelong gluten-free diet is currently the only treatment.

Both companies viewed the early end to the PIVOT-PO trial as a positive development, with GSK saying it would work with US regulatory authorities to move the treatment forward in 2025.

In discussions at INTERPHEX 2025, company representatives seized upon several common themes, most notably safety, sustainability, and the increasing utility of automation.

Global scale-up requires developing replicable processes that work the same no matter where they are performed. This can be accomplished with smart factories that utilize fully automated manufacturing platforms.
Christy Eatmon of Thermo Fisher Scientific reviewed her company’s experience at CPHI Americas 2025 and discussed recent industry trends that are driving strategic partnerships.
MHRA approves Loba Biotech to manufacture Pegasys API, enabling pharma& to strengthen UK supply and ensure continued access for eligible patients.

The concerns of industry experts expressed at INTERPHEX in April 2025 are still pertinent in an uncertain global political climate.

Sanofi is acquiring Vigil Neuroscience, a biotech firm focused on novel neurodegenerative disease therapies, to boost its early-stage neurology pipeline.

Emerson’s purpose-built industrial AI solutions are meant to enhance accessibility and reliability, enabling manufacturers to maximize efficiency and performance from automation systems.

FDA announced efforts to streamline its drug importation process to help U.S. states and Native American tribes access lower-cost Canadian drugs while maintaining safety standards.
In this exclusive Drug Digest video, Adi Kaushal from Lonza summarizes the current state of the oral solid dosage market, identifies the main challenges to drug developers, explains how CDMOs are leveraging expert techniques to improve OSD bioavailability, and looks into the future of advancements in drug delivery.

In this episode of the Ask the Expert video series, Susan J. Schniepp, distinguished fellow at Regulatory Compliance Associates, and Siegfried Schmitt, PhD, vice president, Technical at Parexel, discuss how to address an FDA warning letter citation for a failure to establish a quality control unit.

Mirai Bio and Thermo Fisher partner to integrate AI-driven design with CGMP manufacturing, aiming to streamline development of genetic medicines.

Grifols received FDA clearance to begin a Phase II trial of GRF312, an immunoglobulin eye drop for dry eye disease, aiming to improve current treatments.
Jamie Baumgartner, Jonathan Grinstein, and John Wilkerson go behind the headlines to discuss the implications of personalized gene-editing therapies, more HHS policy and funding updates, and the latest tariff-related investments.
Inobrodib is being developed as a first-in-class oral cancer drug that the company says will be able to treat not only multiple myeloma, but other specific cancers as well.

ACG partnered with Art of You to launch the world-first Personalised Capsule Machine, enabling on-demand, customized supplement capsules based on individual health data.