
Key areas to invest include manufacturing process improvements, quality management, and AI.

Key areas to invest include manufacturing process improvements, quality management, and AI.

In this episode fo the Drug Solutions Podcast, etherna’s vice-president of Technology and Innovation, Stefaan De Koker, discusses the merits and challenges of using mRNA as the foundation for therapeutics in oncology as well as for vaccines.

The non-profit initiative puts collaboration at the forefront as it tackles the challenges surrounding recycling primary pharmaceutical packaging.

The agreement, which will allow participants to share confidential information on medical and medicinal products, builds on the cooperation fostered between the two regulatory authorities during the COVID-19 pandemic.

SK pharmteco, a CDMO, will manufacture, test, and release Adstiladrin (nadofaragene firadenovec-vncg), a gene therapy from Ferring Pharmaceuticals for treating bladder cancer.

The collaboration will combine N4’s nanoparticle delivery system with SRI’s molecular guidance system.

The majority of PMCs/PMRs are proceeding according to schedule.

FDA has granted approval for the use of Novartis’ radioligand therapy, Lutathera, to treat pediatric patients with gastroenteropancreatic neuroendocrine tumors.

In a new agreement, Cellares will utilize its Cell Shuttle fully automated cell therapy manufacturing platform to manufacture select CAR-T cell therapies under development by Bristol Myers Squibb.

A panel of experts went over new regulatory requirements for contamination control and gave guidance on implementing a contamination control strategy in cell and gene therapy facilities at INTERPHEX 2024.

Mary Van Gaasbeck, technical services specialist, LS Equipment and Services, at STERIS Life Sciences, notes that automation is key when it comes to effective sterile powder transfer of parenteral drug products.

Andreas Frerix, product management director for Quattroflow at PSG Biotech, discussed the advantages and new challenges SUTs present for pumping systems.

New regulations, including those put forth by Annex 1, require many pharmaceutical manufacturers to rethink their facility designs to promote compliance.

Jay Rajagopalan, senior director—Engineering & Product Management for Malema at PSG Biotech, shared insights at on how current industry trends are shaping the development and advancement of sensor technologies.

Pharmaceutical Technology® spoke with Nicole Hunter, Watson-Marlow Fluid Technology Solutions’ head of Global WMArchitect, about the impact single-use technologies have on fluid-handling workflows in bioprocessing.

The improvement of ADC manufacturing now demands increased containment requirements and continually advancing analytical detection of molecules in manufacturing spaces.

CordenPharma’s partnership with GENEPEP expedites lead development and validation in project phases, and its LNP β-sitosterol supports sustainable emission reduction targets.

Wenyu Zhang, PhD, addressed new trends in the aseptic industry and the chief concerns companies should keep in mind while weighing their options, at INTERPHEX 2024.

Susan Schniepp, distinguished fellow, Regulatory Compliance Associates, and co-chair of board of directors, Parenteral Drug Association, demonstrates QMS impact on quality maturity at INTERPHEX.

At INTERPHEX 2024, Pharmaceutical Technology sat down with Christa Myers of CRB Group and Nadiyra Walker Speight of Fujifilm Diosynth Biotechnologies to discuss implementation of Annex 1.

The Xcellerex magnetic mixer, single-use mixing system was designed to address challenges in large-scale mAb, vaccine, and genomic medicine manufacturing processes.

Former FDA drug investigator, Daniel Roberts, discusses the importance of updating process validation and maintaining proper data integrity at a keynote session during INTERPHEX.

The airlock solution for cleanrooms offers contamination controls for a highly controlled environment.

At INTERPHEX, Bill Whitford, Strategic Solutions leader at Arcadis, discusses the progress made in 3D bioprinting toward commercial biologics production.

WMFTS has launched WMArchitect, a single-use product line that offers ready-to-use single-use assemblies and custom-designed workflows for biopharma fluid management.
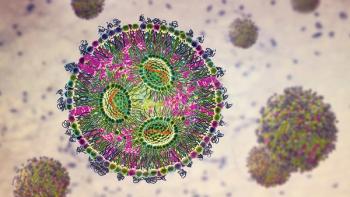
The LNP success in mRNA vaccines leads to their establishment as a standard in mRNA delivery.

C3TI will promote CDER’s clinical trial innovation activities both internally and externally.

The agency’s risk assessment committee has concluded that available evidence has not been found that supports a causal association between glucagon-like peptide-1 receptor agonists and self-harming thoughts and actions.

The draft guidance provides recommendations for data integrity for clinical and bioanalytical portions of bioavailability and bioequivalence studies submitted with drug applications.

Charles River has entered into separate agreements with Axovia Therapeutics and Ship of Theseus to offer plasmid DNA manufacturing services for a gene therapy and a lead candidate program, respectively.