
The human race will have colonized Mars long before the pharmaceutical industry brings itself into the 21st century.

The human race will have colonized Mars long before the pharmaceutical industry brings itself into the 21st century.

A range of insect exterminator equipment has been developed to improve the level of hygiene offered to the pharmaceutical and chemical manufacturing sectors. Berson's Insectron range uses ultraviolet (UV-A) and green light to attract flying insects, which are sensitive to these light sources.

Sigma-Aldrich Fine Chemicals (SAFC) has set up a new business division called SAFC Biosciences to encompass its biopharmaceutical development and cell culture-related manufacturing services.

Biomanufacturing managers believe the current lack of adequately trained personnel is one of the most serious problems facing the biomanufacturing industry.

Lester Crawford, FDA's top man, has resigned explaining that it was time to step down — despite only being promoted to commissioner earlier this year.

FDA's Evans Reviews Causes of Warnings and Recalls

Roche Plans New US Manufacturing Facility for Tamiflu while Cipla Prepares to Market Generic

Patheon, CEPH Begin Corrective Action Program for Omnicef OP

New Legislation To Ensure Stable Flu Supply

USP Announces Monograph Revisions List and Postponed Publication of General Chapter <1226>

Design Exhibition, ?From Master?s Thesis to Medicine Cabinet?

Collaboration Targets Single-Shot Japanese Encephalitis Vaccine

CEPH International Replies to FDA Warning Letter

CDER Creates Office of New Drug Quality Assessment

Ajaz Hussain Leaving FDA for Sandoz
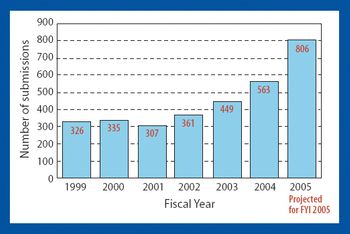
The US Food and Drug Administration (Rockville, MD, www.fda.gov) released a new draft guidance that may speed generic approvals. The guidance, ANDAs: Impurities in Drug Products, describes the degradation-product information that generic drug manufacturers should include in their abbreviated new drug applications (ANDAs). This clarification, FDA officials say, will help companies submit the correct information, thus increasing the likelihood that their generic drugs will be approved, and approved more quickly.

Confronted by a challenge as vast as Hurricane Katrina, we reach for military organizations for aid and military language for description.

It was, our GMP Agent-in-Place recalls, a typical, small, clinical-type facility... managed in the typical, informal way.

As overseas sourcing arrangements become more and more common, it may be time to look again at the advantages of having suppliers closer to home.

One of the most important skills any editor must possess is the ability to listen to their audience — without feedback, we don't get an opportunity to develop our publications; to not act on feedback shows plain ignorance. I must, therefore, give my personal thanks to the many hundreds of readers who participated in the Pharmaceutical Technology Europe (PTE) editorial and readership survey, which we conducted earlier this summer. Your comments and opinions will help us develop PTE, and we already have a number of exciting plans for 2006. Stay tuned!

One of the world leaders in the manufacture of tablet compression tooling, I Holland, is offering a range of machines for the automatic polishing of tablet compression tooling.

Essentially, RAD is a set of tools…that allows for rapid prototyping and reiterative testing that enable faster development and implementation of new applications.
Adding Braille to pharmaceutical packaging should be less of a challenge with the use of Esko-Graphics' Scope solution. EC Directive 2004/27/CE requires Braille labelling and information to be provided with pharmaceutical products for human use, and companies are scrambling to implement this by 31 October.

Biogen to Cut Jobs

The pharmaceutical science education system must be transformed.

The US National Institute of Allergy and Infectious Diseases (NIAID, Bethesda, MD, www3.niaid.nih.gov) announced it will order two million doses of an avian influenza vaccine from Sanofi-Pasteur (Swiftwater, PA and Lyon, France, www. sanofipasteur.com). In April, the NIAID began a Phase I trial to evaluate the vaccine’s safety and ability to generate immunity against the H5N1 strain of avian flu, an illness that leads to severe disease and possible death in birds and humans.

Technology's new monthly "Agent-in-Place" column distills true-life cautionary tales from the secret files of Control, a senior compliance officer.

Lean is good. Emaciated is something else.

Manufacturers of pharmaceuticals and nutraceuticals can protect their products from moisture and other elements while also enhancing marketability, by adding scent to their packaging. The full line of scented packaging solutions, offered by Süd-chemie, is reported to be ideal for applications that emit an unappealing odour to end users. The packaging can also enhance the marketability of odourless products. The flagship product in the scented solution line is Aroma-Can — a product modelled after the company's desiccant canisters. One-gram canisters will be available in orange and lemon scented variations, and can be inserted at the same high rates of speed as traditional desiccant canisters using standard canister insertion equipment.