
Johnson & Johnson (J&J, New Brunswick, NJ) announced last week that it was in "advanced negotiations" for a potential public offer for the Dutch biopharmaceutical company Crucell (Leiden, The Netherlands).

Johnson & Johnson (J&J, New Brunswick, NJ) announced last week that it was in "advanced negotiations" for a potential public offer for the Dutch biopharmaceutical company Crucell (Leiden, The Netherlands).

US Food and Drug Administration Commissioner Margaret A. Hamburg spoke at the Generic Drug User Fees Public Meeting last Friday, Sept. 17, 2010, about enacting user fees for the generic-drug sector.

The US Food and Drug Administration recently issued a Warning Letter to Bristol-Myers Squibb (BMS, New York) for violations of current good manufacturing practice at the company's Manati, Puerto Rico, manufacturing facility.

The European Medicines Agency (EMA) has discussed the possibility of a global framework for clinical trials at an international workshop, given the fact that the majority of clinical trials for medicines approved in Europe are conducted outside of the EMA's regulatory reach.

The European Commission has issued a last call for REACH (Registration, Evaluation, Authorisation and restriction of Chemicals) registration; an EU initiative targeting companies that import or produce chemicals, which will ultimately impact a number of industries, including pharmaceuticals.

Genzyme Sells Genetics Business; Bausch and Lomb Names Vice-President; and More.

Last week, Lonza (Basel) agreed to support the ongoing development of GlaxoSmithKline?s (GSK, London) biopharmaceutical pipeline by supplying manufacturing capacity for five early-stage monoclonal antibodies.

Bristol-Myers Squibb (New York) has agreed to acquire the biopharmaceutical company ZymoGenetics (Seattle) for an aggregate purchase price of $885 million or $735 million net of cash.

ISPE to Hold Risk-MaPP 2010 Conference

The generic-drug company Actavis (Hafnarfjordur, Iceland) is considering acquiring a 51% stake in the biopharmaceutical company BioPartners Holdings (Barr, Switzerland) from Bioton, a Polish biotechnology company.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the September 2010 edition from Alfa Laval and Gems Sensors and Controls.
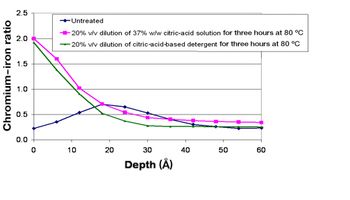
The author describes the types and sources of rouge and explains ways to prevent and mitigate this problem.

US Pharmacopeia apparatuses for testing the dissolution of transdermal drugs produce good, reproducible results. Yet some scientists believe that further modifications could improve the instruments? suitability for this application.

Pfizer to Acquire FoldRx; Protalix Appoints COO; And More.

Roche (Basel) launched a groupwide operational-excellence initiative intended to modify the company's cost structures and accelerate its productivity improvements.

The US Food and Drug Administration launched last week a performance management system designed to advance President Obama's commitment to transparency, public participation, and governmental collaboration.

After hosting the first international scientific workshop on nanomedicines earlier this month, the European Medicines Agency (EMA) has concluded that further research is needed to provide an adequate scientific basis for evaluating the quality, safety, and efficacy of such medicines.

The Society for Chemical Manufacturers and Affiliates (SOCMA) issued recommendations relating to disclosure of confidential business information (CBI) in response to Environmental Protection Agency's (EPA) decision to limit CBI claims on chemical identity under the Toxic Substances Control Act (TSCA).

ICH to Hold Training Workshops for Quality Guidelines

Director General of the European Generic Medicines Association (EGA), Greg Perry, has emphasized the need for EU biosimilar guidelines for monoclonal antibodies (mAbs), as well as a harmonized, global approach to biosimilars in general, at the agency's 8th International Symposium on Biosimilar Medicines.

The European Medicines Agency (EMA) has launched a review of GlaxoSmithKline's pandemic influenza vaccine Pandemrix to investigate whether there is a link between vaccination and cases of narcolepsy, a rare sleep disorder.

Industry efforts toward global healthcare surpass average expectations.

A look at why Brazil revised its GMP standards and how the changes will affect the local pharmaceutical industry.

Pending legislation may give FTC the authority to regulate all Hatch-Waxman settlements.

From weekend deliveries to nonsterile gloves, a single exception can make a product fall flat.

FDA Provides Information On Advisory Committees, Announces Public Meetings, And More.

Industry participation is crucial as USP embarks on far-reaching monograph modernization initiative. This is an online-exclusive article.

New data provide insight into pharma-industry professionals' daily lives.

The President's Council on Advisors on Science and Technology (PCAST), a group administered by the federal Office of Science and Technology Policy (OSTP), issued recommendations to improve the country's ability to accelerate vaccine production and delivery in the event of a pandemic flu outbreak.

Genzyme (Cambridge, MA) confirmed that it had received sanofi-aventis's (Paris) proposal to acquire all of its outstanding shares for $69 each.