
London, UK (Feb. 1)?Researchers at the University of London?s School of Pharmacy have chemically modified carbon nanotubes to enable them to enter human cancer cells.

London, UK (Feb. 1)?Researchers at the University of London?s School of Pharmacy have chemically modified carbon nanotubes to enable them to enter human cancer cells.

Cambrex, Lonza, EaglePicher, more

Washington, DC (Feb. 7)-The Synthetic Organic Chemical Manufacturers Association (SOCMA) submitted comments to the US Department of Homeland Security in response to proposed DHS regulations on chemical site security, asking DHS to take into consideration the unique nature of the specialty batch manufacturing sector.

Washington, DC (Jan. 26)-The US Department of Health and Human Services has granted targeted liability protections for manufacturers of a vaccine to prevent a pandemic of influenza A (H5N1).

New York, NY (Jan. 31)-Pfizer, Inc. scored a victory over Synthon IP in a patent infringement lawsuit over the manufacturing process for ?Norvasc? (amlodipine).

St. Louis, MO, (Feb. 8)-SAFC, the custom manufacturing and fine chemicals unit of Sigma-Aldrich Corporation, is seeking to build a footprint in Asia, eying possible acquisitions in India and China.

Rockville, MD (Jan. 30)-The White House proposes a record $2 billion budget for FDA for the year that starts Sept. 30, 2007, an amount that consists of $1.6 billion in appropriated funds, plus some $450 million user fees for drugs, biologics, medical devices and other products.

Osaka, Japan (Feb. 2)-Tanabe Seiyaku Co. Ltd. and Mitsubishi Pharma Corporation agreed to merge, with the closing planned for merger scheduled to take effect Oct. 1, 2007.

The injunction blocks rules that required secondary wholesalers to supply pedigrees tracing the chain of custody to the maker.

We, the people who make drugs, are extraordinarily good at what we do.

The role of micro-biological testing in real-time release is too important to ignore.

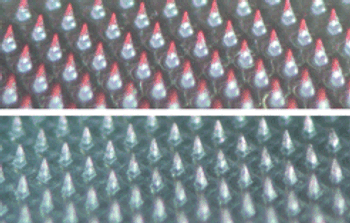
It's what's on the outside that counts, too.

When it comes to ethics, the adage "hindsight is 20/20" is especially applicable. Countless medical and psychological experiments-such as the 1932 Tuskegee syphilis study or Zimbardo's 1972 Stanford mock-prison experiment-were conducted in the name of science and are now plainly recognized as enormous violations of ethical and human rights.


Tokyo (Jan. 18)-Tanabe Seiyaku Co. Ltd. confirmed it has had discussions with Mitsubishi Pharma Corporation over a possible merger between the two companies, but that no definite decision has been made for such a deal.

Rockville, MD (Jan. 30)-The Food and Drug Administration?s Center for Biologics Evaluation and Research (CBER) is proposing an addendum to its current biologics reporting form to better classify potential recalls.

London (Feb. 1)-AstraZeneca PLC unveiled a plan to improve asset utilization within its global supply chain that involves rationalizing production assets and cutting staff. The company made the announcement as part of its fourth quarter and full-year 2007 financial results.

Rockville, MD (Jan. 30)?-In response to a set of recommendations made by the Institute of Medicine, the US Food and Drug Administration issued a report detailing a series of initial steps to improve its safety programs.
Following the sales of its global branded pharmaceutical business, 3M stays the course in drug delivery as its Drug Delivery Systems Division advances technology in inhalation and transdermal drug delivery.

Cardinal Health (www.cardinal.com) agreed to sell its contract services unit, Pharmaceutical Technologies and Services (PTS), to The Blackstone Group (New York, NY, www.blackstone.com) for roughly $3.3 billion in cash.

The US Department of Health and Human Services (HHS, Washington, DC) has awarded three vaccine makers a total of $132.5 million to advance their strategies for adjuvant-containing vaccines to combat the H5N1 strain of avian influenza. Under the contracts, each company will build capacity to produce either 150 million does of the vaccine or enough adjuvant for 150 million doses within six months after the onset of an influenza pandemic.

The US Food and Drug Administration announced the creation of the Office of the Chief Medical Officer as well as two important staff changes at the agency.



Indianapolis, IN (Jan. 11)-Eli Lilly and Company announced several strategic changes to its global manufacturing operations. The changes include termination of construction of a planned insulin manufacturing plant in Virginia, staff reductions in its operations for small-molecule active pharmaceutical ingredients (APIs), and investments in manufacturing biotech-based drug products

Rockville, MD (Jan. 12)-To prevent the spread of bovine spongiform encephalopathy (BSE) and related diseases, the US Food and Drug Administration has proposed banning certain cattle tissues and tissue-products from the manufacture of drugs for human and ruminant use. Though cattle products are used in 75% of pharmaceutical processes and 90% of biotechnology processes, the Agency says that no approved or investigational drug appears to contain bovine material that would be prohibited under the rule.

Brussels, Belgium (Jan. 11)-EPCglobal, the not-for-profit organization dedicated to driving global adoption of the Electronic Product Code (EPC), has ratified the electronic pedigree document specification.

Akorn, Alkermes, Eli Lilly, Cambrex, more

Washington, DC (Dec. 28)-The US Department of Homeland Security (DHS) has proposed regulations for improving security at high-risk chemical facilities, a category that may include some pharmaceutical production facilities.

Darmstadt Germany (Jan. 8)-Merck KGaA closed on its roughly CHF 16.6 billion ($13.3 billion) deal to acquire a majority stake in the European biotechnology company Serono (Geneva, Switzerland), officially launched Merck Serono SA as a new entity within Merck KGaA, outlined its integration strategy, and announced plans to divest its generics business.