
Interphex FasterCures Keynote Speaker Stresses Communication and Collaboration

Interphex FasterCures Keynote Speaker Stresses Communication and Collaboration

Bayer Bids for Schering AG in Friendly Merger Offer

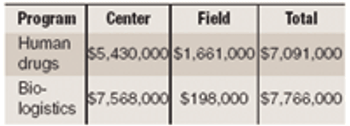
Fundamentals Stay Strong for Pharma Contract Services Industry

FDA's Risk-based Model Leads to Strategies for Optimizing Site Risk Potential

Merck KGaA Launches Bid For Schering AG

Bush Administration Nominates von Eschenbach to head FDA

Chiron Withdraws Measles Vaccine

Roche Expands "Tamiflu" Production

Novasep Divests Swiss Fine Chemicals Producer Rohner AG

Watson To Acquire Andrx for $1.9 Billion

Development Begins on Mutated H5N1 Virus Vaccine

Proposed Legislation to Help Fight Counterfeit Drugs

Schering-Plough Reorganizes Management in Europe, Latin America and Asia-Pacific

Novavax, Bharat Biotech Team for Pandemic Influenza Vaccine Development

Eisai Plans $105 Million Pharma Production and Formulation R&D Facility

BMS Plans to Build $660 Million Bulk Biologics Manufacturing Facility
Flu vaccines. Dow veterinary vaccine made in plants. $1.95 billion FDA budget focuses on high-profile programs. Non-injectable insulins win approval. FDA releases guides to dissolution testing, quality overall summary, and new drug-label formats.
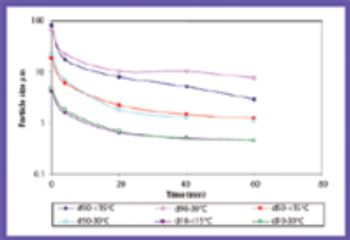
A modified modular high-pressure system can form nanosuspensions of model compounds by individually controlling cavitation, impact, and shear forces.

P&G Pharmaceuticals Plans Job Cuts

Chiron and Novartis Advance Programs for Pandemic Flu

Madness in March: lost ingredients, missed lot numbers, and a million-dollar photo.

Chiron To Sell Betaseron Assets to Schering

Letter to the Editor: Glen Jon Smith
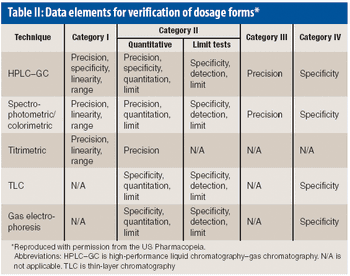
USP's proposed General Chapter 1226 "Verification of Compendial Procedures" aims to provide guidance about the verification process.
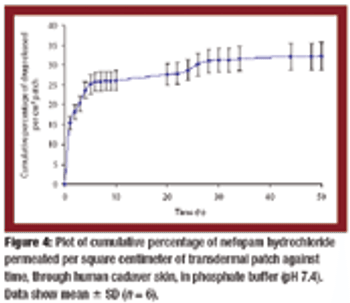
Transdermal matrix-type patches of Nefopam hydrochloride with a combination of pressure-sensitive adhesives were developed. The polymeric composition provided a controlled and sustained release of the drug from the patches and demonstrated favorable physicochemical characteristics.

BMS Receives FDA Approval for Transdermal Antidepressant

Novartis Plans Manufacturing Site in China

FDA's 2007 budget request funds homeland security and pandemic preparedness by reducing support for the agency’s core mission.

Secondary sensors help protect feedback control systems from the effects of sensor drift.