
Seeking more than $5.8 million in damages and the recovery of nearly $1.8 billion in punitive damages, RxUSA Wholesale (Port Washington, NY) filed a complaint against 16 major US pharmaceutical manufacturers and 5 drug wholesalers.

Seeking more than $5.8 million in damages and the recovery of nearly $1.8 billion in punitive damages, RxUSA Wholesale (Port Washington, NY) filed a complaint against 16 major US pharmaceutical manufacturers and 5 drug wholesalers.

Dietmar Hopp, cofounder of the German information technology giant SAP AG (Waldorff, Germany) is forming a new pharmaceutical company from the merger of two German biopharmaceutical companies: Axaron Bioscience AG (Heidelberg, Germany) and Lion Bioscience AG (Heidelberg, Germany).

Novartis (Basel, Switzerland) will build a cell culture-derived influenza vaccines manufacturing plant in Holly Springs, North Carolina. Construction is expected to begin in 2007.

In a move to strengthen its position in Western generic drug markets, Ranbaxy Laboratories Ltd. (Gurgaon, Haryana, India) acquired the Mundogen generic drug business of GlaxoSmithKline (GSK, London, England) in Spain, through Ranbaxy's Spanish subsidiary, Laboratorios Ranbaxy S.L.

Vincent L. Vilker, Ph.D., has been appointed director of FDA's Office of Testing and Research (OTR). Vilker was formerly chief of the Biotechnology Division at the National Institute of Standards.

The US Food and Drug Administration today posted its final "Guidance for Industry: Providing Regulatory Submissions to the Center for Biologics Evaluation and Research (CBER) in Electronic Format: Lot Release Protocols."

AAI Pharma, Alexion Pharmaceuticals, Altana, Athenagen, Cardinal Health, Nektar, Roche

A group of researchers from Georgia Institute of Technology (Atlanta, GA) are using high-throughput ionization techniques to identify and measure the ingredients in counterfeit drugs.

Although the number of anti-infective vaccines (as distinct from therapeutic vaccines for cancers and other noninfectious diseases) entering clinical study each year since 2000 has been higher on average than it was in the 1990s, this product area may see little additional growth through the rest of this decade, according to a recentanalysis from the Tufts Center for the Study of Drug Development (Boston, MA).

Late last week, Pliva d.d. (Zagreb, Croatia) announced that the US Food and Drug Administration had granted final approval for its warfarin sodium tablets and azithromycin for oral suspension.

The Bayer Group (Leverkusen, Germany) plans to sell the diagnostics division of Bayer HealthCare to Siemens AG (Munich, Germany) for EUR 4.2 billion ($5.36 billion).

The US Food and Drug Administration (Rockville, MD) announced that Baxter Healthcare Corp. (Deerfield, IL) signed a consent decree relating to the company's "Colleague" volumetric infusion pump and "Syndeo" patient-controlled analgesic syringe pump.

Vaccine maker Sanofi Pasteur, Inc. received a US Food and Drug Administration Warning Letter, dated June 30, citing "significant deviations" from current good manufacturing practices in the production of monovalent concentrates used in the company?s ?Fluzone? influenza vaccine.

Abbott Labs, Cambrex, Cardinal Health, Charles Ross and Son Co., Janssen, West Pharmaceutical Services

Dutch biotechnology company Crucell NV (Leiden, Netherlands) and its technology partner DSM Biologics BV, a business unit of Royal DSM NV (Heerlen, Netherlands) will open a new research and development center that will specialize on further developing the "PER.C6" human cell line for the expression of recombinant pharmaceutical proteins.

Does the competition from authorized generics really help lower drug prices and boost healthcare savings?
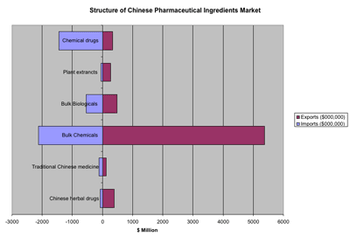
The sixth CPhI China exhibition, presented June 27?29 in Shanghai, offered a showcase for the explosive growth of the Chinese pharmaceutical sector.

University of Buffalo (Buffalo, NY, www.buffalo.edu) researchers have developed a drug delivery system that uses an external magnetic field to guide drug-filled nanocarriers to cultured tumor cells.

MedImmune, Inc. (Gaitherburg, MD) reports that US Food and Drug Administration (Rockville, MD) has approved the company's supplemental biologics license application to use reverse genetics technology to construct new vaccine strains to produce seasonal influenza vaccines.

Biopharmaceutical company Lipoxen PLC (London, UK) has developed a Hepatitis E vaccine using its novel vaccine delivery technology "ImuXen," which the company claims to be easy to manufacture. According to the company, the proprietary liposomal formulation method delivers vaccine materials to the immune system in a manner designed to emulate the response of a natural encounter with the infection agent.

From RFID and pedigrees to PAT and process understanding, industry professionals are eying the growing gap between ambitious high-level guidance and current practice, and wondering how they can bridge this new space and keep in compliance with the untested rules.

A cleverly developed patent portfolio can block or minimize competition long past a patent's 20-year lifespan.

Pharmaceutical Science & Technology News

My audit host clearly knew and lied about the planned changes.

We can learn a lot about today's regulatory environment as a whole if we take it all in.

China is on the rise as a center for pharmaceutical R&D, but companies are still getting their footing for operating in China and the services industry has some maturing to do.

The creation in the Paris Region of a multidisciplinary Institute of Technology will bring together the best researchers in world, innovative SMEs and the research centres of large industrial groups.
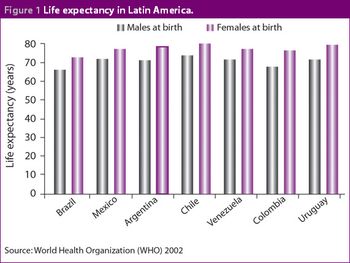
Although it has recently been surpassed by Mexico in terms of value, the Brazilian pharmaceutical market remains of key importance to companies establishing themselves in Latin America. The Brazilian government has focused heavily on improving the healthcare system and this should lead to long-term benefits for its citizens. The Ministry of Health has also attempted to decentralize the management of the healthcare system to more regional and local levels. This has been necessary to account for the different healthcare priorities in different parts of the country.

Last week, the Generic Pharmaceutical Association (GPhA Arlington, VA) praised a proposal by the Senate Agricultural Appropriations Subcommittee that, if approved, would provide $10 million in additional funding for the US Food and Drug Administration?s (Rockville, MD) Office of Generic Drugs.

Baxa Corporation, Eli Lilly, PLIVA, SkyePharma, Wyeth