
The collaboration between Parexel and Partex is designed to leverage artificial intelligence-powered solutions to accelerate drug discovery and development.

The collaboration between Parexel and Partex is designed to leverage artificial intelligence-powered solutions to accelerate drug discovery and development.

FDA granted accelerated approval to Elrexfio (elranatamab-bcmm) for adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy.

The agreement also lists that AGC Biologics will leverage the company’s monoclonal antibody development and manufacturing experience to perform process transfer, process optimization, and clinical manufacturing of the Asahi Kasei drug substance.

HitGen has entered into a research service agreement with Nested Therapeutics for use of HitGen’s DNA-encoded library for drug discovery.

The launch of Alpha Teknova’s new manufacturing facility in Hollister, Calif., increases its capacity for custom reagents for life sciences applications.

Gilead and Tentarix established three multi-year collaborations to discover and develop multi-functional, conditional protein therapeutics for oncology and inflammatory diseases.

CPHI 2024 will return to the Fiera Milano in Milan, Italy.

Zygel is the first and only pharmaceutically manufactured, synthetic cannabidiol that is formulated as a patient-protected permeation-enhanced gel for transdermal delivery.

The acquisition of the latter includes the company’s lead development asset INV-202, an oral CB1 inverse agonist which is designed to block the receptor protein CB1.

The completion of three acquisitions of Versanis Bio, Sigilon Therapeutics, and DICE Therapeutics boosts Eli Lilly and Company’s product pipeline in obesity, diabetes, and immunology.

Under the exclusive agreement, Hikma will commercialize products in Rakuten Medical’s pipeline for cancer treatment in the Middle East and North Africa region.

A report from the UK Bioindustry Association report indicated that biotech venture and public financing rose from £295 million in the first quarter to £382 million in the second quarter.

Cytomos will use the £4 million in funding to develop its dielectric spectroscopy technology.

Additionally, Poseida has approved Astellas as a board observer seat, which gives Astellas the right to attend Poseida’s scientific advisory board meetings and certain notice rights related to any potential change of control over Poseida.

Novartis has completed its acquisition of biopharma company Chinook Therapeutics.

The guidance can help both applicants and manufacturers limit the mutagenic and carcinogenic potential of NDSRIs.

The new drug could be a powerful tool for patients suffering depressive symptoms after birth.

The site was acquired by AGC Biologics in July 2020, and the Milan location is the first cell and gene therapy site approved in Europe for GMP manufacturing of clinical and commercial supplies.

The initial collaboration between the two companies began in 2017 with an extension announced in 2021.
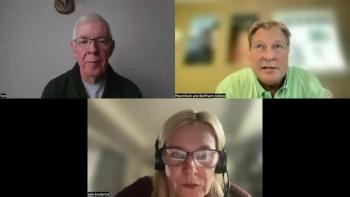
Highlights from a wide ranging discussion on COVID-19 pandemic response from Kate Broderick, Chief Innovation Officer at Maravai LifeSciences, and Tom Madden, President & CEO at Acuitas Therapeutics.

SBS-518 is a first-in-class dual sigma receptor (SR) antagonist/dopamine transporter (DAT) inhibitor in development for the treatment of stimulant use disorder (StUD).

The investigational drug was developed to activate a novel uptake pathway, allowing antineoplastic drugs to combat solid tumors more successfully.

The bioreactors can integrate with Emerson’s DeltaV PK Controller in addition to S-Series and M-Series controllers.

Lower levels of an active ingredient may result in unexpected pregnancies.

BlueRock will be responsible for the global development and commercialization of therapeutic candidates emerging from this collaboration.

Under a manufacturing agreement, Northway Biotech will develop a manufacturing process for iTolerance’s fusion protein in development for regenerative therapy.

Waters XBridge Premier GTx BEH size exclusion chromatography (SEC) columns are designed to improve analysis while lowering the cost of gene therapies.

Kiefel’s new machine is designed to form, fill, and seal up to 6,000 infusion, parenteral nutrition, or dialysis bags per hour.

The nasal-spray drug can be used to reverse the effects of opioid overdose.

Laboratories are focusing on more automated processes while also trying to maintain an efficient, safe, and personalized experience with each analytical test.