
Consider modular automation and cybersecurity when modernizing manufacturing processes.

Consider modular automation and cybersecurity when modernizing manufacturing processes.

Edge-AI technology will enable Industry 4.0 automation in pharmaceutical manufacturing.

Automation and tools for remote monitoring are finding increased use as pharmaceutical manufacturing adapts to pandemic restrictions.

Ongoing and emerging cyber-risks to legacy operational technology systems in bio/pharmaceutical manufacturing must be addressed.

The register supports cross-functional teams of chemists and biologists in developing non-natural, chemically-modified therapeutics for drug discovery.

Honeywell Forge Cybersecurity Suite’s improved industrial-grade remote access and added protection and risk monitoring aids remote operations.

Quartic.ai and Bright Path Laboratories have agreed to develop an artificial intelligence (AI)-powered continuous manufacturing platform for APIs and other small-molecule drugs using Bright Path Labs’ continuous bioreactor and the Quartic.ai smart manufacturing technology.

Remote, machine health monitoring services reduce the need for on-site visits.

MilliporeSigma’s Bio4C Software Suite combines process control, analytics, and plant-level automation.

A comprehensive turnkey system based on LabVantage’s laboratory information management system platform allows laboratories to implement COVID-19 biobanking, testing, and research.

Analyzing process and equipment data provides insights that can improve quality and productivity of pharmaceutical manufacturing.

Enhancements to Augury’s AI-based machine health platform include new capabilities and collaboration tools for personnel working remotely.

Emerson’s Mimic Field 3D program offers an immersive simulation using a digital twin as an alternative to training on physical equipment.

Vein-to-vein programs are focusing on data access and traceability.

CDMOs must consider challenges associated with the complexity of contract pharmaceutical manufacturing when approaching digitalization projects.

Data collected through the Industrial Internet of Things enable predictive maintenance.

Sartorius and the German Research Center for Artificial Intelligence have established a research laboratory for AI in the biopharmaceutical industry.

Predictive and prescriptive maintenance improve pharmaceutical manufacturing equipment effectiveness.

In batch production, efficient exception management means reducing the time required to identify, review, and resolve process exceptions. Incorporating review by exception functionality within manufacturing execution system (MES) software can streamline biopharmaceutical product release.

The pressure to improve operational technology performance combined with the rapid pace of technology change is driving adoption of new automation models in pharmaceutical manufacturing.

Systems-based pharmaceutics case studies, challenges, and new uses were discussed at PSE’s Advanced Process Modeling Forum.

The Industry 4. 0 tools of augmented and virtual reality aid pharmaceutical fill/finish equipment design, training, and troubleshooting.

Digital tools, such as building information modeling (BIM) software, improves efficiency for optimizing pharmaceutical facility design.
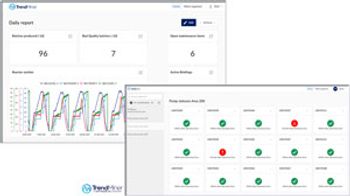
TrendMiner 2019.R3 displays a customized, actionable dashboard with a real-time view of the production process.

Machine vision systems have been an integral part of pharma manufacturing and packaging for many years and, with the introduction of stricter safety regulations, are set to become more vital.