
Companies know they need regulatory information management, but they don't always know where to start and how to weave this capability across diverse technology applications.

Companies know they need regulatory information management, but they don't always know where to start and how to weave this capability across diverse technology applications.

President Obama unveiled an Advanced Manufacturing Partnership designed to reinvigorate the country's manufacturing sector.

The Association of the British Pharmaceutical Industry (ABPI) has published a guidance that suggests best practices for managing adverse events and other pharmacovigilance data from the internet and social media tools.

FDA added a searchable database of inspection data to its website. The database lists the names and addresses of facilities that the agency inspected during fiscal years 2009 and 2010.

In January 2011, a new version of Annex 11 was released by the European Commission along with a revision of Chapter 4 of its GMP on documentation to reflect the actualities of electronic record keeping, all of which come into full effect in June 2011.

In January 2011, the European Medicines Agency (EMA) announced the new revision of EudraLex Volume 4 (GMP) - Annex 11 'Computerised Systems', and the consequential amendment of EudraLex Volume 4 - Chapter 4 ?Documentation?.

Can the semiconductor industry help Big Pharma develop therapies?
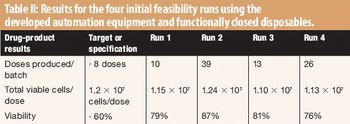
The authors developed automated equipment that uses functionally closed disposables to perform cellular and ribonucleic processing.

Axcan acquires Mpex Pharmaceuticals; Bend Research receives patent for improving bioavailability of low-solubility drugs; and More.

Eli Lilly and GlaxoSmithKline are among several pharmaceutical companies using technology transfer as way to improve health outcomes in the developing world.

Pfizer Completes King Acquisition; ViiV Healthcare Names Chairman; and More.

FDA recently sent sanofi-aventis two Warning Letters for its facilities in Frankfurt am Main, Germany, and Bridgewater, NJ.

INTERPHEX 2011 aims to address the industry's unique characteristics.

Gilead to Acquire Calistoga; Bayer Healthcare Appoints Former Pfizer Exec; and More.

Company and People Notes: sanofi to Acquire Genzyme; Lilly Makes Senior Appointments; and More.

Pfizer Acquires Ferrosan's Consumer Health Business; Patheon Appoints Former Biogen Exec as CEO; and More.

The European Medicines Agency has released a new Annex 11-Computerized Systems to its GMP Guide Chapter 4 on Documentation to account for the increased use of and complexity of computerized systems in the drug-manufacturing community.

Company and People Notes: Pfizer Completes King Tender Offer; Cook Pharmica Names VP of R&D and CSO; and More

Follow-ons were all the rage at this year's JP Morgan Healthcare Conference.

Novartis Acquires Genoptix; Lilly Exec Becomes Savient CEO; and More.

Merck Teams with Parexel; Roche CFO to Retire; and More

Gilead Sciences to Acquire Arresto Biosciences; Sartorius Appoints Head of Lab Business; and More.

Capable of great works, pharma as a whole still yields to the lesser angels of its nature.

AstraZeneca and Abbott End Program; PhRMA Elects New Members; and More.

Pfizer Recalls One Lipitor Lot; Sanofi Aventis Names Head of R&D; and More.