
Pharma 4.0 envisions highly efficient automated processes, which could be continuous, batch, or a hybrid of these, driven by an integrated manufacturing control strategy.


Pharma 4.0 envisions highly efficient automated processes, which could be continuous, batch, or a hybrid of these, driven by an integrated manufacturing control strategy.

Sensors and devices being developed by nGageIT Digital Health Solutions can track patient use of oral solid-dosage or injectable drugs.

Can an Irish analytics company and its CEO bring pharma closer to 21st-century practice?

Automation systems, vehicles, and robots improve efficiency of transporting materials and finished goods in pharmaceutical warehouses.

Honeywell offers an augmented/virtual reality approach to train the industrial workforce.

The Industrial Internet of Things and new tools such as smart glasses can improve training and daily tasks for pharmaceutical manufacturing operations.

Automated Control Concepts has launched Lab Owl bioreactor control system for labs using cell culture and fermentation applications.

BioTek Instruments has released a second edition of its BioSpa software that now offers users a simplified but effective interface for kinetic imaging or detection workflows.

A survey by LNS Research and sponsored by Honeywell showed that industrial companies are not moving quickly to adopt cyber security; calls on CEOs to take action.

Supply Chain Wizard has developed a set of integrated digital solutions to improve supply chain functions.

The Human Vaccines Project has created the Universal Influenza Vaccine Initiative, a research program that will aim to understand the human immune system’s role in the development of universal influenza vaccines.

The M&A advisory firm has developed an interactive map of manufacturing sites to give insight into the market size of global manufacturing.

The International Society for Pharmaceutical Engineering announced the release of ISPE GAMP Good Practice Guide: IT Infrastructure Control and Compliance (Second Edition).

The International Society of Automation (ISA) and Siemens entered a global partnership to increase awareness of industrial cybersecurity needs and standards.

Everyone from IT departments through to manufacturing line personnel should be aware of cybersecurity threats and how to prevent attacks.

Artificial intelligence offers a number of opportunities in pharmaceutical drug development and manufacturing, but there are barriers to overcome, notably around how well and how quickly the regulatory environment can adapt and keep pace with to the rapid changes.

Electronic systems allow biopharma manufacturing companies to meet data integrity requirements and optimize practices.


A staggering percentage of people do not take their medication correctly, but pharmaceutical packaging aims to improve patient compliance using new technology to address reasons for non-adherence.

The annual meeting of the Honeywell Users Group for the Americas will discuss industrial automation.
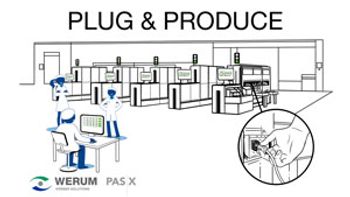
Werum’s Plug & Produce solution eases connection with smart equipment and control systems.

The author shares his opinion on the challenges presented by the Internet of Things and what companies need to consider when choosing suitable architectures to manage serialization data.

Samsung BioLogics’ aggressive growth strategy begs the question: Are there lessons that US and European pharma might still learn from the electronics industry?

A rigorous approach to industrial security is essential for protecting intellectual property and product integrity in connected pharmaceutical operations.

igital tracking of overall equipment effectiveness can improve efficiency.