
In the pharmaceutical factory of the future, data collected by internet-connected manufacturing equipment improves operational efficiency.

In the pharmaceutical factory of the future, data collected by internet-connected manufacturing equipment improves operational efficiency.

InstantGMP PRO 3.0 software integrates production scales with an electronic batch record system.

A new version of Siemen’s Simatic EBR software offers web-based master batch records and integration with automation systems.

Designing systems using the principles of good documentation practice, including validated audit trails, is a key piece of a manufacturing data integrity program.
Computerized systems can solve some of the data integrity problems with conventional paper-based systems.

Advanced electronic batch recording (EBR) manages workflows and recordkeeping for compliance and production efficiency.

Integrated data and cloud-based solutions can be used for process optimization.

Smart glasses enhance remote troubleshooting and process management for pharmaceutical manufacturing.

Near-field communication labels communicate with consumers and support personalized medicine.

Smart glasses enhance remote troubleshooting and process management for pharmaceutical manufacturing.
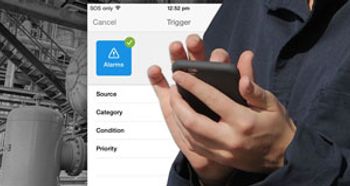
Honeywell Process Solutions’ Honeywell Pulse app brings notifications, real-time plant performance data, and analytics directly to mobile devices.

Rockwell Automation’s updated PlantPAx DCS enhances automation and control.

Designed specifically for pharmaceutical companies, the online production management system focuses on plant operations so materials flow smoothly to help companies achieve right-first-time manufacturing and faster product release.

Applications in the Rockwell Software Studio 5000 development environment add functionality for automation engineers.

Everyone talks about “open innovation,” but without the right working culture it won’t succeed. MeetingZone’s Anthony Prior explains how unified communications can help kick-start the necessary change.

The system is suited to support quality-control activities in a busy laboratory.

Employing continuous manufacturing and data analytics will help improve the efficiency of pharmaceutical manufacturing in the facility of the future.


Under a global strategic partnership, Siemens will become GSK’s preferred supplier of manufacturing automation, focusing on process control, equipment control, and building management systems.

Virtual pilot programs that simulate scenarios can help the pharmaceutical industry address core issues in the implementation of serialization systems that comply with the US Drug Supply Chain Security Act.

MES has already rewarded early adopters by reducing errors; next-generation technology will enable broader data analysis.

Honeywell’s latest release of Experion LX, a distributed control system designed specifically for small- and mid-sized companies, enhances batch, SCADA, and engineering capabilities.

Paperless operations improve efficiency and increase assurance of product quality.

Systec & Solutions' HMI hardware and Werum's PAS-X manufacturing execution system are designed to work together.

The new Kaye Validator AVS combines accurate sensor measurements with all GMP requirements for calibration and traceability.