
The European Organisation for Rare Diseases (EURORDIS) has predicted that 100 new designated orphan products will receive marketing authorization between 2009 and 2019, which equates to approximately 10 new products every year.

The European Organisation for Rare Diseases (EURORDIS) has predicted that 100 new designated orphan products will receive marketing authorization between 2009 and 2019, which equates to approximately 10 new products every year.

The author details the factors in formulation design, requirements in facilites and equipment, and validation criteria for aseptic formualtions.

The author discusses the risks involved with aseptic processing, methods and tools used to identify and control risk, and regulatory guidelines relevant to the risk-management process.

Mark Copley discusses the methods used for DPI testing and the challenges presented by the current regulatory framework.

An inhaler mouthpiece that optimizes drug delivery to the lungs and reduces the amount of wasted medication has been developed by US researchers.

The global API market has expanded from $69 billion in 2004 to more than $90 billion in 2008 at an average yearly growth rate of 7.2%, according to a report from the Italian Chemical Pharmaceutical Association (CPA).

Cheaper generic pharmaceuticals, emboldened by US law and patent challenges, restrict novel future innovations.

For the first time, cancer treatments accounted for 5% of overall drug spending in the first half of 2009, said Medco Health Solutions in a press release last week.

GlaxoSmithKline (GSK), Novartis, sanofi aventis and Baxter International have provided updates regarding the development, manufacture and shipment of pandemic (H1NI) vaccines.

A new drug delivery method developed by scientists could enable prescription drugs to be buried inside the body where drug release could be prompted by a biological trigger, such as a drop in blood sugar levels, or activated manually with a pulse of light.

A long-term joint venture between GlaxoSmithKline (GSK) and China's Jiangsu Walvax Biotech Company hopes to develop and manufacture paediatric vaccines for use in China.

GlaxoSmithKline (London), Novartis (Basel, Switzerland), sanofi Aventis (Paris), and Baxter International (Deerfield, IL) recently provided updates as to the development, manufacture, or shipment of pandemic (H1NI) vaccines.

Company and People Notes: GSK forms joint venture with China-based Jiangsu Walvax Biotech; Sigma-Aldrich appoints VP and board member; more...
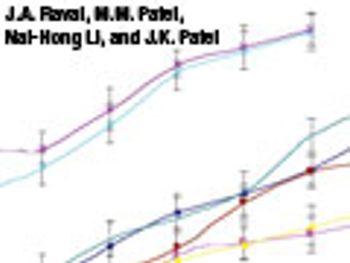
The authors investigated the effects of formulation and processing parameters on floating matrix-controlled drug-delivery systems.

BIO supports recent Congressional action toward a 12-year data exclusivity period for innovators.

The author discusses control strategies via near infrared instrumentation for continuous mixing, granulation, drying, and extrusion with a more focused detail on mixing.

Two vaccines manufactured by Novartis and GlaxoSmithKline (GSK) against influenza H1N1 have been recommended by the EMEA for marketing authorization.

Formoterol presents formulators and manufacturers in the asthma and chronic obstructive pulmonary disease marketplace many challenges.

On September 25, the European Medicines Agency's (EMEA) Committee for Medicinal Products for Human Use (CHMP) recommended the authorization of two vaccines for use in Europe against the H1N1 influenza: GlaxoSmithKline's (GSK) Pandemrix and Novartis's Focetria.

A joint venture between a charity and a pharma giant has led to the creation of the Hilleman Laboratories, which will use a not-for-profit operating model to develop and deliver vaccines to low-income countries.

The US Food and Drug Administration approved on Tuesday four H1N1 flu vaccines that demonstrated in clinical studies that a single dose produced a strong immune response in healthy adults after 8?10 days. But clinical trials of the vaccine are still underway on pregnant women and children, two groups that the Centers for Disease Control and Prevention (CDC) says are especially vulnerable to the H1N1 flu.

Semisolid Formulation Development: The CRO Approach - SP Formulations Whitepaper

The FDA has approved four vaccines for the H1N1 influenza virus, while other companies, including UK pharma giant GlaxoSmithKline (GSK), have reported promising results from clinical studies with a single dose vaccine.

The US Food and Drug Administration has approved four vaccines for the H1N1 influenza virus, while other companies, including GlaxoSmithKline (GSK), have reported promising results from clinical studies with a single dose vaccine.

Generic-drug companies are increasingly viewing the development of controlled-release formulations as a way of obtaining a competitive edge, according to a report published by Espicom Business Intelligence in late August 2009.