Although realized in 1983, ASTM's standrad test method for determining bacterial retention of membrane filters used for liquid filtration is still hugely beneficial to today's pharmaceutical industry.

Although realized in 1983, ASTM's standrad test method for determining bacterial retention of membrane filters used for liquid filtration is still hugely beneficial to today's pharmaceutical industry.

During its roundtable discussion titled "Follow-On Biologic (FOB) Drugs: Framework for Competition and Continued Innovation," The Federal Trade Commission (FTC) discussed likely effects, patent issues, and regulatory exclusivity periods concerning FOBs.

Optimizing the solid form of a drug reaps scientific and technical awards.

A well-balanced guide to industrial bioseparations provides valuable information.

Despite challenges, contract manufacturers in Europe are enjoying considerable success.
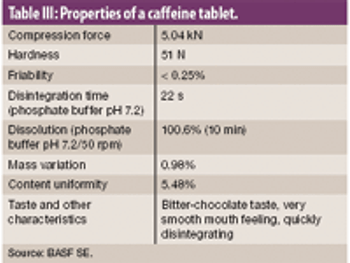
The authors examine the effectiveness of an excipient comprised of mannitol, polyvinyl acetate, and crospovidone using model actives loperamide hydrogen chloride and caffeine.
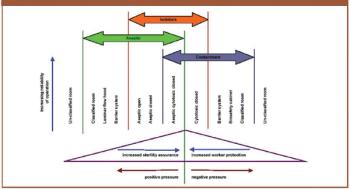
The author discusses the key issues to consider when using isolators such as containment, protection of personnel, the efficiency of biodecontamination cycles, sterility assurance levels, barriers and their integrity, and environmental impact.
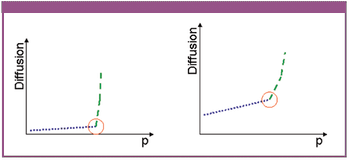
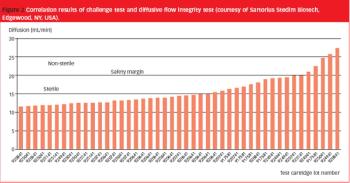
The authors describe a novel approach for the integrity testing of large sterile filter systems such as multiround housings and describe a multipoint diffusion test capable of detecting minor failures.
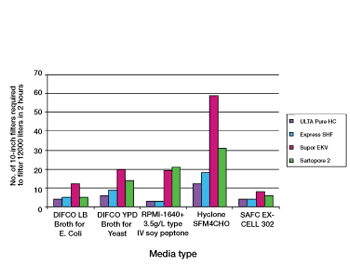
Proper selection of normal flow filters leads to increased process efficiency from early phase product development through to full-scale biopharmaceutical production.

A comparison of conventional cleanrooms, restricted access barrier systems, and isolators, shows the benefits of using isolators in high-potency drug manufacturing.
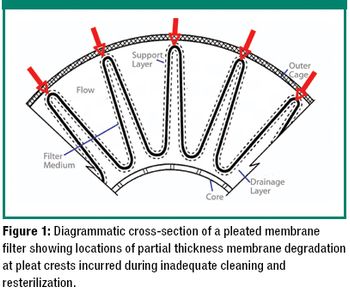
The author examines re-use of hydrophilic- or hydrophobic-membrane sterilizing-grade filters in liquid sterilizing applications.

More than 235 biotechnology companies call New Jersey home. They range from early start-ups with just a few employees to fully functioning companies with research, marketing and sales offices.

Although California remains the country's biotech leader, Michigan anticipates that the wind is shifting direction, albeit slowly

There are many challenges upstream and downstream in manufacturing a biotech drug.

Richard Kilner, Managing Director of the Commonwealth of Pennsylvania European Investment Office, is incredibly enthusiastic about both his State and the potential it offers investors: "We are the number one State in pharmaceuticals (by number of establishments, employment and GDP output) and rapidly closing in on Massachusetts, the number two in biotech."

Access to the capital markets of the US has always been a key attraction for Europe's biotech businesses.

Many compounds fail in preclinical development because of safety-related problems, but identifying 'predictable' safety or toxicity liabilities earlier in the process could lead to improved design and selection of compounds that are more likely to be approved.

California's forward-thinking reputation, well-funded research universities and world leadership in the potentially life-saving field of stem cell research and green energy provide a progressive and positive business environment for biotech companies.

Speaking to Pharmaceutical Technology Europe, Georgia Bio's Director of Innovation and Technology, Carol Henderson, outlined the unique assets that make Georgia an attractive location for international bioscience companies.

According to Deputy Director, Alexander Bothmann, of Enterprise Florida Inc., in Germany, the 'Sunshine State' is: "Committed to building a world-class biotechnology sector by investing in research facilities, fostering the growth of local biotech companies and welcoming progressive newcomers, such as the Scripps Research Institute, the Burnham Institute for Medical Research and Torrey Pines Institute for Molecular Studies."
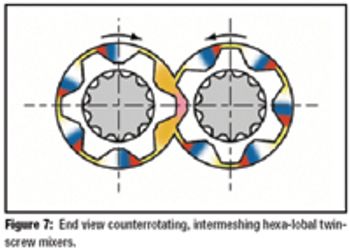
The author describes the benefits, processes, and practicality of using hot-melt extrusion to mix active pharmaceutical ingredients with pharmaceutical-grade polymers.
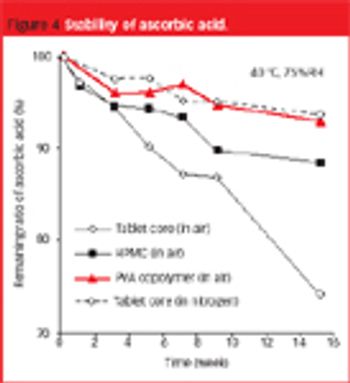
The use of PVA copolymer-based film can solve the problems associated with lack of film adhesion... to tablets containing large amounts of waxy excipient or a lubricant.
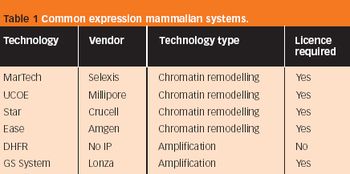
...an uninformed decision based on commercial requirements alone may, ultimately, have disastrous consequences on the efficacy of the target recombinants.

FDA has issued a Final Rule titled "Amendments to the Current Good Manufacturing Practice Regulations for Finished Pharmaceuticals."

USP announced an interim revision to its monograph for levothyroxine sodium tablets, which will become official in USP 32-NF 27.