
Why correctly calibrating a drug with its packaging is the key to success.

Why correctly calibrating a drug with its packaging is the key to success.

The Czech Republic will no longer purchase the H1N1 flu vaccine from Baxter International.

Scientists have modified a tobacco plant to produce a vaccine for norovirus, the viral infection sometimes referred to as the "cruise ship virus".

Scientists have modified a tobacco plant to produce a vaccine for norovirus, the viral infection sometimes called the "cruise ship virus."

NIR Prediction of Solid Dosage Form Dissolution Profiles - Foss Whitepaper

Extending Calibrations for Near-Infrared Assay of Tablets Using Synthetic Modeling and Variance from Placebos - Foss Whitepaper

Cooling water is a critical component in the research and development, bulk manufacturing, and packaging of pharmaceuticals.

Baxter International (Deerfield, IL), sanofi aventis (Paris), and Novartis (Basel, Switzerland) provided updates last week of their production and regulatory activities relating to preparedness in supplying the A(H1N1) pandemic influenza vaccine. Novartis also outlined its activities for providing seasonal flu vaccines.
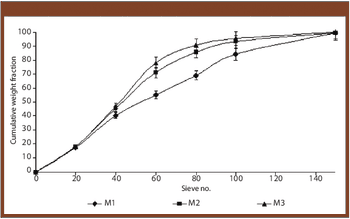
The authors prepared granules containing cinnarizine using polyethylene glycol 6000 as a melting binder and lactose monohydrate as hydrophilic filler. The effects of binder concentration and size were studied.

Two years ago, the US Food and Drug Administration's Office of Generic Drugs (OGD) developed and implemented a program to test question-based reviews for abbreviated new drug applications (ANDAs). OGD is reviewing the benefits and challenges faced by pharmaceutical firms involved in the initiative to see what changes need to be made going forward.

The Federal Trade Commission (FTC) last week issued an interim report that examined the effects of authorized generics on competition in the prescription drug market.
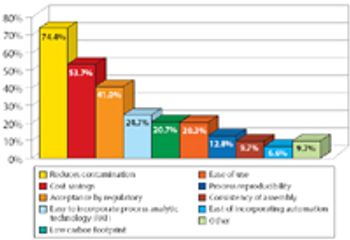
A Pharmaceutical Technology report looks at trends in biopharmaceutical manufacturing. This article contains bonus online-exclusive material.
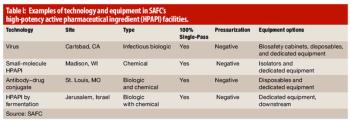
High-potency manufacturing of active pharmaceutical ingredients is a growing and specialized capability.
The authors discuss how strategic outsourcing to contract manufacturing organizations that have technical and regulatory expertise can add further value during vaccine development.
Biopharmaceutical companies generally try to avoid paying royalties on novel technologies, including novel expression systems, in part because of the inability to predict revenue flow after a product is commercialized.

The emergence of influenza A (H1N1) and the efforts to provide vaccines to the vulnerable are timely examples of biopharmaceuticals' continuing importance.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.

To address the increasing popularity of drug-injector systems, the US Food and Drug Administration last week released a Draft Guidance for Industry titled, Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products.

Bulk Inspection of Tablets-Symetix Applicaton Note
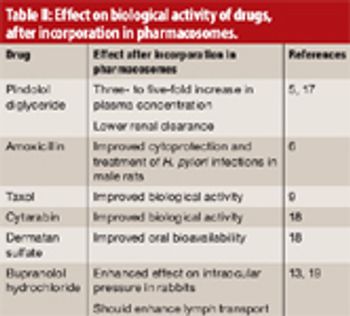
Pharmacosomes can pass through biomembranes efficiently and possess several advantages over traditional vesicular drug-delivery systems.

There may well be a pending revolution in biopharmaceutical expression systems. Nearly 50% of biomanufacturers today are demanding a whole lot more from their primary expression systems than they have during the past 30 years.
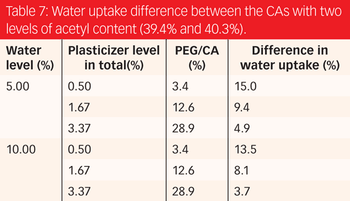
Understanding a formulation's variable effects on its properties, especially film permeability, is key to designing a robust formulation and reducing variation of a finished product.
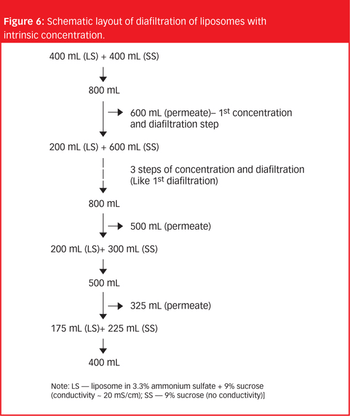
The importance of liposomes as an effective drug delivery system is well accepted in the pharmaceutical industry, but their handling remains a challenge.

The Question-Based Review (QbR) initiative of the Office of Generic Drugs has reached its second full year in 2009. Special from the Journal of Validation Technology.

The United States Department of Health and Human Services (HHS) this week placed an initial order with Sanofi Pasteur (Lyon, France) for a vaccine to fight influenza A (H1N1) infection.