
FDA Clears Infant Rotavirus Vaccine

FDA Clears Infant Rotavirus Vaccine

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the May 2010 edition from ATMI and SciLog.

In response to stringent price cuts introduced in Spain, the European Generics Association (EGA) is urging the Spanish government to adopt mechanisms that will help increase the dispensing of generic medicines.

The traditional lines of demarcation between innovator-drug and generic-drug companies are blurring as each sector responds to changing industry fundamentals.

Further consolidation, slowing growth in established markets, and growth in smaller and emerging markets are important factors influencing the direction of the global generic-drug market.

The authors demonstrate that sustained-release delivery can help avoid the risk of sudden higher-blood concentration of a drug to avoid toxicity.
The author outlines the key concepts of ISPE's recently revised Baseline Pharmaceutical Guide for New and Renovated Facilities. This article is part of a special supplement on Excipients and Solid Dosage.

Testing the dissolution rates of a pharmaceutical formulation (also known as in vitro availability) aids drug quality control and is a compulsory requirement of the British, European and US pharmacopoeias.

A new automated factory in Boston (MA, USA) has been developed that uses non-genetically modified green plants to quickly produce large quantities of vaccines and therapeutics.

After several years of debate and review, the US Food and Drug Administration is calling for the removal of metered-dose inhalers.

Tablets and capsules are mainstay product forms, so what are the spending and innovation trends for solid-dosage manufacturing equipment and machinery?

In Part I of this article, which appeard in the March 2010 issue, the authors describe their approach for constructing form spaces for carbamazepine, cimetidine, and phenylbutazone by initial solvent screening to evaluate the feasibility of spherical crystallization. Part II of this article discusses their findings.

The authors describe the origins of single-use components and explain their application to aseptic processes. They also show how disposable devices have changed over time and offer a glimpse of the future.

Could insect cells offer a faster way of manufacturing pandemic influenza vaccines compared with traditional egg-based methods? According to researchers at the Vienna Institute of BioTechnology (Austria), their new technique could help a virus-like particle (VLP) vaccine to reach the market within 3 months from the first isolation of a new influenza strain - traditionally produced vaccines take approximately 6 months.

Vibrational spectroscopy, which encompasses near-infrared, mid-infrared and Raman spectroscopy, is an important analytical tool in the pharma industry.

Florian Krammer explains how a novel technology using insect cells can accelerate the manufacture of pandemic influenza vaccines.

Three companies joined the University of Pittsburgh Medical Center's 21st Century Biodefense initiative to establish a flexible vaccine development and production facility.
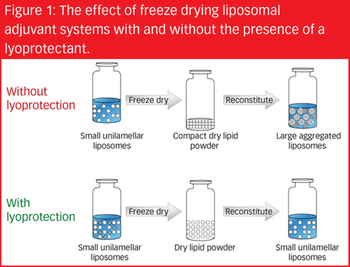
There are a variety of vaccine types, each varying in safety and efficacy, and each possessing its own formulation challenges. To overcome potential instabilities when developing vaccines, one formulation strategy is to produce a dried product.

The US Food and Drug Administration recently published guidance for the characterization and qualification of cell substrates, viral seeds, and other biological materials used to manufacture viral vaccines for human use.
In Part I of this article, the authors describe the materials and methods used in developing a screening strategy to accelerate the preparation and characterization of spherical agglomerates by spherical crystallization.

Analysis of the opportunities and challenges in the biosimilars market.
Technological developments make it easier to manufacture sterile parenterals.
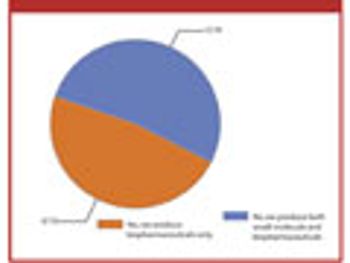
The second annual Pharmaceutical Technology Bioprocessing Survey offers a snapshot of the industry following 2009's megamergers.

Drugmakers have many incentives to avoid overfilling their containers, including the scarcity, and correspondingly high cost, of certain cells and ingredients. These concerns highlight the need for techniques that can fill small volumes of product with great accuracy. Many strategies are available to the industry, but which one works best?

Figures published by the European Medicines Agency (EMA) relating to centralized procedure activities for human medicines show a significant increase in the number of positive opinions made between 2007 and 2009; however, the majority of approvals are for generic products.