
The authors demonstrated that ODTs can be obtained by direct compression of a mixture of starch, fructose, and SMCC.

The authors demonstrated that ODTs can be obtained by direct compression of a mixture of starch, fructose, and SMCC.

The author reviews key considerations for formulating powders for use in inhalers. This article is part of a special Drug Delivery issue.

A review on the current status of long-acting injectables, including commercially marketed products. This article is part of a special Drug Delivery issue.

The authors describe an alternative approach to compressing and coating minitablets for use in a sustained-release, solid oral-dosage form. This article is part of a special Drug Delivery issue.

The author outlines how to choose carriers and capsule shells according to dosage requirements and intended use. This article is part of a special Drug Delivery issue.
The author reviews advances in technology that may soon allow transdermal delivery of two of the fastest growing drug classes on the pharmaceutical market. This article is part of a special Drug Delivery issue.

Although there are no regulatory requirements or established pharmacopoeial techniques for the dissolution testing of inhaled drugs, such testing can potentially open up the opportunity to tailor formulation properties.

Pfizer announced this his week that it had entered into a strategic global agreement for the worldwide commercialization with the Indian biotechnology company Biocon (Bangalore) for biosimilar versions of insulin and insulin analog products (e.g., recombinant human insulin, glargine, aspart, and lispro).

FDA to Hold Public Hearing on Biosimilars Legislation, And More.

A recently released industry guide outlines a science- and risk-based approach to control the risk of cross-contamination.
Drugmakers hatch new manufacturing paradigms in the wake of the 2009 H1N1 influenza pandemic.

A look at MVI's malaria work in developing countries.

Novartis' Matthew Stober discusses vaccine manufacturing, including egg- and cell-based systems.

President Obama and HHS eye innovation and countermeasures to protect public health.

The early part of the decade saw a decline in vaccine sales and manufacture, but finally the industry is bouncing back, with Europe particularly well placed to make an impact.
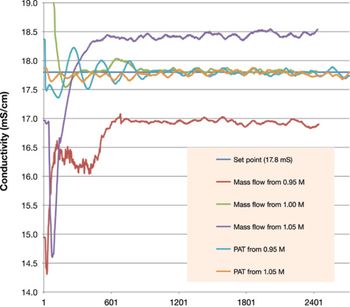
The authors describe the operational requirements and design of a process-ready PAT-based IBD system.

The author discusses new detection-system technologies that can improve performance and provides key criteria to consider when selecting or upgrading a system for pharmaceutical use.

The generic-drug company Actavis (Hafnarfjordur, Iceland) is considering acquiring a 51% stake in the biopharmaceutical company BioPartners Holdings (Barr, Switzerland) from Bioton, a Polish biotechnology company.
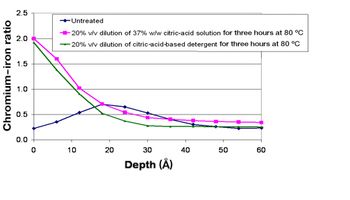
The author describes the types and sources of rouge and explains ways to prevent and mitigate this problem.

US Pharmacopeia apparatuses for testing the dissolution of transdermal drugs produce good, reproducible results. Yet some scientists believe that further modifications could improve the instruments? suitability for this application.

Director General of the European Generic Medicines Association (EGA), Greg Perry, has emphasised the need for EU biosimilar guidelines for monoclonal antibodies (mAbs), as well as a harmonised, global approach to biosimilars in general, at the agency's 8th International Symposium on Biosimilar Medicines.

Director General of the European Generic Medicines Association (EGA), Greg Perry, has emphasized the need for EU biosimilar guidelines for monoclonal antibodies (mAbs), as well as a harmonized, global approach to biosimilars in general, at the agency's 8th International Symposium on Biosimilar Medicines.

The European Medicines Agency (EMA) has launched a review of GlaxoSmithKline's pandemic influenza vaccine Pandemrix to investigate whether there is a link between vaccination and cases of narcolepsy, a rare sleep disorder.

The President's Council on Advisors on Science and Technology (PCAST), a group administered by the federal Office of Science and Technology Policy (OSTP), issued recommendations to improve the country's ability to accelerate vaccine production and delivery in the event of a pandemic flu outbreak.

A Strengths, Weaknesses, Opportunities and Threats analysis of the biosimilars market is given.