
This injection formulation of USP-grade leucovorin calcium is now available in the United States.

This injection formulation of USP-grade leucovorin calcium is now available in the United States.

The US and pharmerging markets will drive growth in drug spending; number of approvals to increase.

Lonza and Takeda have furthered their multiproduct partnership through collaborative efforts on a new cancer treatment-Alunbrig (brigatinib).

The agreement gives Sanofi access to Biomunex’s proprietary bi- and multi-specific-antibody-generating platform.

Sanofi’s Adacel (tetanus toxoid, reduced diphtheria toxoid and acellular pertussis [Tdap] vaccine adsorbed) is approved for a repeat dose in people 10 through 64 years of age, 8 years or more after the first vaccination.

The new reagents are designed to support clinical-phase and commercialization stages of cell and gene therapy production and to enhance DNA transfection.

AbbVie and immunotherapy company Tizona Therapeutics will join forces to develop and commercialize CD39-targeted therapeutics to treat cancer.

The company has brought a second stream of high-throughput GMP peptide manufacturing online.
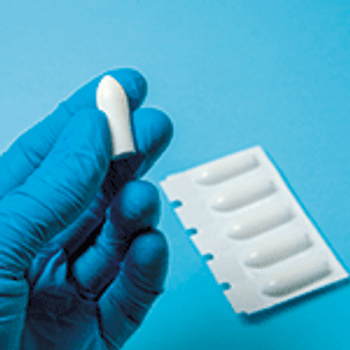
This article demonstrates how qualitative physical attributes testing can be used to characterize soft gel capsule rupture/disintegration during rectal administration.
New approaches seek to address formulation and delivery challenges for these complex molecules.

Teva Pharmaceuticals and Neos Therapeutics entered into a settlement and licensing agreement to resolve all ongoing litigation involving Neos' patents protecting its Cotempla (methylphenidate) extended-release orally disintegrating tablets.

Agilent and other partners are funding development of Tapestri, a single-cell sequencing platform designed to help predict cancer relapse in individual patients and show the efficacy of gene-editing experiments.

The use of artificial intelligence creates growth opportunities in novel therapeutics development by leveraging multi-sourced data, according to experts at research and consulting firm Frost & Sullivan.

The company’s biosimiliar to Roche’s Avastin (bevacizumab) received a positive opinion for marketing authorization from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP).

Avacta Group and LC Chem Life Sciences have entered into a multi-target collaboration and development agreement.

FDA has withdrawn the proposed rule that would have allowed generic-drug makers to independently update and distribute new safety information in drug labels.

FDA has approved Truxima (rituximab-abbs), a biosimilar to Roche’s anti-cancer biologic, Rituxan (rituximab).

GSK and TESARO have entered into a definitive agreement concerning GSK's acquisition of TESARO for an aggregate cash consideration worth approximately $5.1 billion.

AbbVie will grant Pfizer license to launch a biosimilar to AbbVie’s Humira worldwide.

Transdermal patch design, materials, and manufacturing variables, as well as drug formulation and interactions between the API and the adhesive, can affect adhesion and drug delivery.

Virpax’s Patch-in-a-Can technology delivers pain medication using a metered-dose spray film.

Drug and adhesive formulation are crucial to the development of microneedle patches for pharmaceutical transdermal delivery systems.
Antibody-based drugs offer new mechanisms of action and greater specificity.
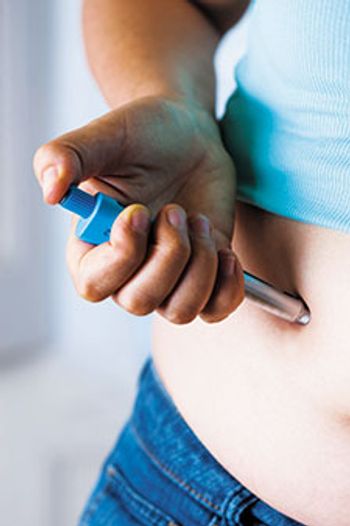
Using training devices may ease patient anxiety about using autoinjectors and prefilled syringes, potentially leading to improved patient adherence.

FDA Commissioner Scott Gottlieb has been promoting drug market competition in recent months that includes new guidance documents and targeted advisories to support R&D of complex drugs and combination products.