
Moving forward with gene therapy development requires a “quantum leap” in manufacturing capabilities.

Moving forward with gene therapy development requires a “quantum leap” in manufacturing capabilities.

The testing of raw materials is essential as raw material quality determines the outcome of biologic product quality.

The new program will provide cell and gene therapy companies a more efficient way to ensure quality compliance across collection center networks and to minimize quality system audit burden on these centers.

Permission from the United Kingdom's Supreme Court has been granted to the UK BioIndustry Association, allowing it to intervene in a dosage regimen patent case.

Medherant has announced positive results from two Phase I trials evaluating its transdermal drug delivery patch loaded with ibuprofen.

Formulation issues cause significant drug project delays and project failures, according to a 2018 survey conducted by Informa Pharma Intelligence, Rentschler Biopharma, and LEUKOCARE.

AbbVie will grant Momenta license to launch a biosimilar to AbbVie’s Humira.

Researchers evaluated the chemical stability of two different naloxone products administered to prevent opioid overdose deaths and found the products to have a significantly extended shelf life than previously known.

Irish manufacturer of generic medicines for both human and animal health, Chanelle Pharma, has been named as Pharma Industry Company of the Year 2018.

The company received FDA approval for Hyrimoz (adalimumab-adaz), its biosimilar referencing AbbVie’s blockbuster Humira (adalimumab).
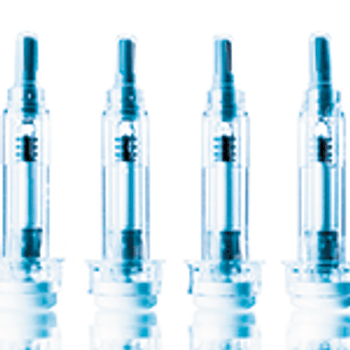
As biologic drugs grow increasingly complex, drug delivery mechanisms such as prefilled syringes are being adapted to meet the challenges.

Demand for highly potent APIs continues to rise.

he acquisition expands the company’s formulation and analytical R&D services.

The companies announced the European launch of Imraldi (adalimumab), a biosimilar referencing AbbVie’s blockbuster Humira (adalimumab).

Mylan announced the launch of its biosimilar to AbbVie’s Humira across major European markets.

To achieve a more dynamic marketplace, FDA is issuingguidance documents and targeted advisories to support R&D on complex generics and combination products.

Amgen’s biosimilar to AbbVie’s Humira (adalimumab) is the first inflammation biosimilar from Amgen's portfolio to launch in Europe.

Cambrex will create a new center of excellence for API process development and clinical supply at its High Point, NC facility and expand its API manufacturing facility in Italy.

The company’s planned investment in its alkoxylation facility in the United States Gulf Coast will also expand production capacity for its polyethylene glycols.

The company will collaborate with GlycoBac to offer an insect cell line for the development of viral vaccines and gene therapies.

Novartis’ Sandoz has reached a global patent resolution for Hyrimoz (adalimumab), its biosimilar to AbbVie’s Humira (adalimumab).

A new investigational vaccine, LASSARAB, shows promise for use against Lassa fever and rabies.

The company says the new equipment will reduce sample turnaround times and increase variant detection quality and accuracy.

Advances in biologic drug development require increased methodological and technological innovation from the biopharma industry. Learn more at the new bioLIVE launching this year adjacent to CPhI Worldwide 2018.

Lonza’s Capsugel Colorista capsule R&D solution can cut overall development time and offers greater flexibility during technical color development.