
Increasing dwell time can improve tablet production.

Increasing dwell time can improve tablet production.
Development costs and time to market continue to put pressure on the biopharma industry, driving the need for innovation in methods and technologies.
Determining how much containment is needed for API handling requires evaluation of multiple factors.

API can be mixed with silicone and other polymers to create drug-delivery combination products.

CMOs have been active over the past year in expanding their biologics production and capabilities.

The recommended drugs include two orphan medicines and three biosimilars.

The company has run into a snag in producing Kymriah for the diffuse large B-cell lymphoma patient population, the second indication for which the therapy was recently approved by FDA.

In launching FDA’s Biosimilar Action Plan, Gottlieb takes innovator companies to task for delaying competitive biosimilar products.

AbbVie will grant Mylan license to launch a biosimilar to AbbVie’s Humira.

CELLforCURE will produce cancer CAR-T treatments for Novartis at a manufacturing facility in Les Ulis (Essonne), France.

The media, by Tosoh Bioscience, is composed of calcium and phosphate buffers and offers mixed-mode properties for biomolecule purification.

The agency is releasing six new draft guidances to provide a regulatory framework for handling gene therapies.

Pfizer creates separate business units for innovator medicines, generic drugs, and consumer products.

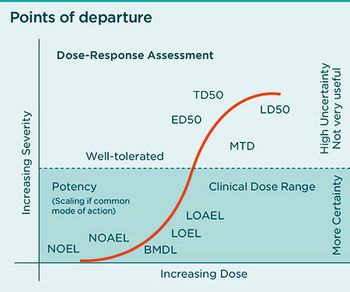
Report predicts PAT, NIRS, continuous bioprocessing, and a ‘technological arms race’ could improve biopharma manufacturing efficiencies.

Biogen will pay Samsung BioLogics approximately $700 million to increase its stake in Samsung Bioepis to approximately 49.9%.

Bosch Packaging Technology’s GKF 720 capsule filling machine for small batches of hard capsules uses a fully automated, washable containment process.

After 30 years of biologic-drug advances, the industry and patients still have a lot to learn.
Biopharma seeks alternatives that meet the needs for next-gen biologic drug production.

With the right excipients, formulators can control when, where, and how an API is released.
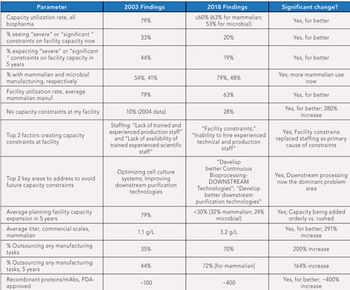
This article highlights 15 years of changes in biopharmaceutical manufacturing.

FDA seeks more efficient testing to spur development of less costly biotech therapies.

Macromolecular drugs are typically injected, but oral dosage forms are being developed to improve the treatment of gastrointestinal conditions such as inflammatory bowel disease.

A new oral delivery method developed by the Harvard John A. Paulson School of Engineering and Applied Sciences could change the way diabetics regulate blood sugar levels.

Minakem’s facility in Belgium enhances capacity to scale production of highly potent ingredients for small to full GMP batches.

The company has completed the first phase of expansion at its headquarters in Freiburg, Germany, in anticipation of increasing demand as cell and gene therapies approach commercialization.