
The new features on the purifier deliver additional sizes and sterilization/sanitization compatibility.

The new features on the purifier deliver additional sizes and sterilization/sanitization compatibility.

The collaboration will focus on developing manufacturing solutions for biosimilars.

Bio-Rad introduces CHT Ceramic Hydroxyapatite XT media and Nuvia HP-Q resin resin for process protein purification.

New products were developed as next-generation process intensification technologies, MilliporeSigma reports.

The company is increasing manufacturing capacity at its Copenhagen, Denmark facility with the addition of six new bioreactors.

A collaboration integrates Sartorius Stedim Biotech’s BIOSTAT STR bioreactors and Repligen’s XCell ATF cell-retention control technology to create simplified, scalable equipment for intensified cell culture.

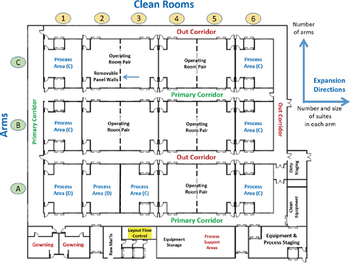
A multi-purpose biopharmaceutical manufacturing facility using a matrix of multi-functional cleanrooms can be adapted to efficiently meet the capacity challenges of both supplying clinical trials and launching products.

The SciLog SciPure FD System from Parker Bioscience is an automated single-use system for the filtration and dispensing of products into either bottles or bags.

Single-use technologies, modular systems, and robots are on the rise.
Immobilizing the antibodies on a solid-phase support, such as a resin, and carrying out the conjugation of the payload-linker while the antibodies are bound to that support will prevent aggregation at its source.

The company is certified as a manufacturer of pressure vessels and components for use in China.

The new Testa Center in Uppsala, Sweden is a collaborative test bed offering biotechnology equipment from GE Healthcare for process development.

Dompé Farmaceutici’s Oxervate (cenegermin), a topical eye drop, is the first treatment approved by the agency for neurotrophic keratitis, a rare disease affecting the cornea.

The company’s purpose-built facility in Norwich, United Kingdom specializes in plant-based expression of proteins, metabolites, and complex natural products.

FDA grants support US research in continuous manufacturing monitoring and control techniques for bio/pharmaceutical manufacturing at Rutgers, MIT, and Georgia Tech.

Drug product approval from FDA follows previous approvals from European and Japanese authorities.

The new drug, Onpattro (patisiran), by Alnylam Pharmaceuticals, is in a new class of drugs called small interfering ribonucleic acid (siRNA) treatment.

Avalon and Weill will co-develop bio-production and standardization procedures for CAR-T therapy.

The companies will collaborate on the discovery, development, and commercialization of cell therapies for cancer.

Boehringer Ingelheim joins Oxford BioMedica, UK Cystic Fibrosis Gene Therapy Consortium, and Imperial Innovations to form a partnership for developing a new gene therapy to treat cystic fibrosis.

The company broke ground on its $200-million, 120,000-ft2 biomanufacturing plant in West Greenwich, RI.
Modular manufacturing systems offer a less costly way to increase capacity while reducing time-to-market.
Development costs and time to market continue to put pressure on the biopharma industry, driving the need for innovation in methods and technologies.

CMOs have been active over the past year in expanding their biologics production and capabilities.