
Moderna’s new manufacturing plant in Norwood, MA gives the company capacity for preclinical and Phase I and II clinical manufacturing for its mRNA development candidates, including personalized cancer vaccines.

Moderna’s new manufacturing plant in Norwood, MA gives the company capacity for preclinical and Phase I and II clinical manufacturing for its mRNA development candidates, including personalized cancer vaccines.

The agency is releasing six new draft guidances to provide a regulatory framework for handling gene therapies.

Report predicts PAT, NIRS, continuous bioprocessing, and a ‘technological arms race’ could improve biopharma manufacturing efficiencies.

The acquisition of Flex Concepts adds custom, single-use products to Entegris’ single-use bag product line.
Biopharma seeks alternatives that meet the needs for next-gen biologic drug production.
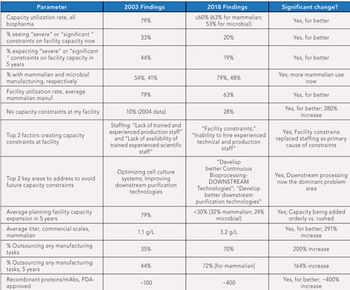
This article highlights 15 years of changes in biopharmaceutical manufacturing.

Macromolecular drugs are typically injected, but oral dosage forms are being developed to improve the treatment of gastrointestinal conditions such as inflammatory bowel disease.

The new resin used a combination of “jetting” technology and a high-performance Protein A ligand.

The company’s next-generation ultraperformance liquid chromatography platform is designed to meet the evolving laboratory requirements for chromatographic performance.

The company has completed the first phase of expansion at its headquarters in Freiburg, Germany, in anticipation of increasing demand as cell and gene therapies approach commercialization.

The contract development and manufacturing company has received an additional approval from Health Canada to manufacture monoclonal antibody drug substance at its first plant in Icheon, South Korea.

WuXi Biologics will invest $60 Million to establish a biologics production facility in Massachusetts.

The company will provide the first FlexFactory manufacturing platform for cell therapy manufacturing.

Flow imaging microscopy can be used to identify particulates and their sources.

Bosch Packaging Technology will present its new modular freeze dryer at Achema 2018.

GE Healthcare and the Centre for Commercialization of Regenerative Medicine (CCRM) will support scale-up efforts by DiscGenics for a new cell therapy intended to treat back pain.

This marks the third FDA approval for the company’s second biomanufacturing plant in Incheon, Korea.

The European Commission has approved Zessly (infliximab), a biosimilar to Johnson & Johnson’s blockbuster Remicade (infliximab).

The gene therapy company is expected to invest $55 million in a new manufacturing facility that will produce therapies for rare neurological genetic diseases.

In adding a Vanrx Pharmasystems aseptic filling isolator, FUJIFILM adds fill/finish for gene therapies and viral vaccines.

WuXi Biologics will build a biologics manufacturing facility in Singapore that will use both fed-batch and continuous perfusion-based single-use bioreactors.

The new company will develop proprietary RNA-based therapeutics and will provide broad lentiviral development and manufacturing expertise and support.

The company is increasing its cell culture media production capacity at its facilities in Pasching, Austria, and Logan, Utah.

Aerobic bioprocesses are highly dependent upon the oxygen transfer rate (OTR) from sparged to dissolved gas. The relatively low solubility of oxygen, however, makes the choice of mixer impeller configuration a critical design factor for the bioreactor vessel. This article describes a series of experiments and computational fluid dynamics (CFD) simulations to detail the effect of mixer configuration on the efficiency and effectiveness of a bioreactor vessel with respect to blend time and mass transfer.

Advanced data analytics tools are used by industry to find the golden nuggets in historical data, to aid in process development, to fine-tune production, and to achieve long-term improvements in product quality and throughput.