
Janssen Pharmaceuticals, Inc. and GlycoVaxyn AG have entered into a three-year agreement to collaborate on the research and development of a multivalent bacterial vaccine utilizing GlycoVaxyn's bio-conjugation technology.

Janssen Pharmaceuticals, Inc. and GlycoVaxyn AG have entered into a three-year agreement to collaborate on the research and development of a multivalent bacterial vaccine utilizing GlycoVaxyn's bio-conjugation technology.
As 2012 comes to a close, Pfizer leads among Big Pharma companies for FDA approvals of new molecular entities and biologics.

Novartis announced that it has received FDA approval for a seasonal influenza vaccine produced in cell culture, the first seasonal vaccine produced by this method to be approved in the US.

The Italian Medicines Agency lifted a temporary hold on the use of Novartis seasonal influenza vaccines in Italy after affirming the vaccines' safety and efficacy.

Policymakers must balance fundamental issues involving access to medicines and pricing.

A Q&A with James Ingebrand, Vice President and General Manager of 3M Drug Delivery Systems Division, on recent industry trends.
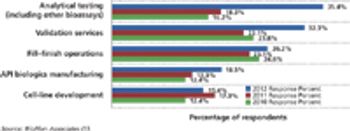
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.

MedImmune, the global biologics arm of AstraZeneca, announced that it is restructuring its infectious disease and vaccines R&D and operations. This will result in the closure of MedImmune's sites in Mountain View and Santa Clara, California.

Novartis, GlaxoSmithKline, and Lonza are among those participating in newly formed consortiums for developing and producing medical countermeasures.

Dr. Reddy's Laboratories and Merck Serono, a division of Merck KGaA, have partnered to codevelop a portfolio of biosimilar compounds in oncology.

BIO is calling for a more patient-centric approach to user-fee reauthorization.

FDA issued a final rule on sterility testing on May 3, 2012, which amends the requirements for most licensed biological products and aims to provide manufacturers with the flexibility, as appropriate, to keep pace with technological and scientific advances. Many steps are changed or eliminated.
The author discusses potential opportunities to improve the patient experience through formulation and delivery device technologies.
Readers share their views on bioprocessing challenges, equipment use, and outsourcing trends in our annual bioprocessing equipment and processing survey.

Collaboration can begin with a conversation.

There are no two completely identical freeze dryer units in operation anywhere.

GlaxoSmithKline and Daiichi Sankyo have formed a joint venture that they claim will create the biggest vaccines company in Japan. The joint venture will seek to improve access to vaccinations in the Asian nation as well as introduce new vaccines.

The standardization of upstream and downstream bioprocessing is growing, but several kinks need to be ironed out.

Pharma announces plans for the year ahead at annual JPMorgan Global Healthcare conference.

We bring industry experts together to discuss the importance of self-administration and what injection technologies are best suited to this cause.

Pfizer and GlaxoSmithKline have announced separate agreements with the GAVI Alliance to supply pneumococcal vaccines to developing countries. Pneumococcal disease can lead to pneumonia, meningitis, and sepsis, and is one of leading causes of death in children under the age of five in developing countries.

Pfizer and GlaxoSmithKline have announced separate agreements with the GAVI Alliance to supply pneumococcal vaccines to developing countries.

Last week, the US Department of Health and Human Services and Novartis Vaccines and Diagnostics dedicated a manufacturing plant that can create influenza vaccine using cultured animal cells instead of the conventional expression system of fertilized eggs.

EMA released two concept papers for consultation that address the need to revise existing guidelines on biosimilar medicines and influenza vaccines.
Single-use components in the fill–finish line provides increased flexibility to multiproduct manufacturers.