
A study shows high levels of ADAs to infliximab at the beginning of treatment were associated with a poor response later on.

A study shows high levels of ADAs to infliximab at the beginning of treatment were associated with a poor response later on.
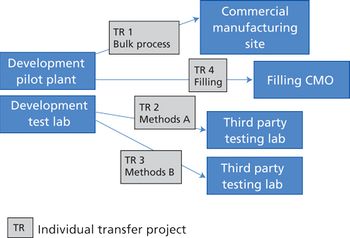
Integrating quality and compliance with technology transfer and careful project management are key in starting up a facility and launching a biologic drug.

Aseptic spray drying provides an alternative to lyophilization as an enabling stabilization technology for parenteral biologic formulations.

MilliporeSigma and the International Vaccine Institute in Seoul, South Korea aim to develop more robust, scalable vaccine manufacturing processes.

Studying broadly neutralizing antibodies in infants may lead to new pathways in HIV vaccine development.

The inactivated vaccine, manufactured with a GE FlexFactory system, could be associated with fewer adverse reactions than the live vaccine option.

The wearable devices for the delivery of biologic products are now being manufactured and will be tested in clinical trials in the near future, according to the company.

Experts discuss the key considerations in the development of an autoinjector.

Collaboration and single-use technologies aided the rapid scale-up of Ebola vaccine manufacturing

MilliporeSigma expands its Carlsbad, California-based GMP capacity for viral and gene-therapy products by nearly 90%.

Sanofi will invest in its Geel, Belgium facility in order to support its pipeline of monoclonal antibodies.

A report by Reuters found that four of the top 10 most widely used drugs increased 100% since 2011, while the other six increased 60%.

Global outbreaks energize vaccine R&D and drive production modernization.

Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.
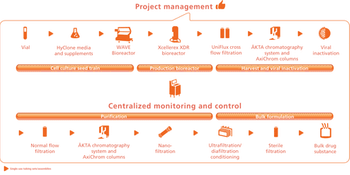
Asking the right questions is crucial to establishing a biopharmaceutical facility design.

Takeda Pharmaceuticals announced the acquisition of a biopharmaceuticals manufacturing plant in Minnesota.

Samsung BioLogics begins construction on their third facility in Songdo, Korea.

Seqirus, CSL Limited’s influenza vaccine business, announced the opening of their corporate headquarters in the United Kingdom.

FDA confirmed quality focus while Congress moved to bolster biomedical innovation.

Biotech boom, niche markets, smaller batch sizes and high potency manufacturing are among the key trends shaping the pharmaceutical industry of the 21st century, according to Christian Treitel from Bosch Packaging Technology.

South Africa’s Biovac Institute, which develops and produces vaccines for the country, launched a public-private partnership with Pfizer to enable local manufacturing of Prevenar 13, a vaccine against pneumonia-causing bacteria.
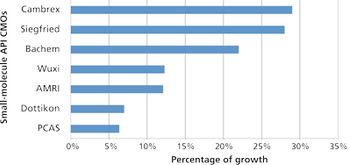
While biologics development grabs investor interest, small-molecule APIs still hold two-thirds of the drug-development pipeline.

According to a third-quarter earnings report, Pfizer’s vaccine business contributed nearly 14% to its total revenue.

Univercells will integrate its single-use bioprocess with the Takeda vaccines production platform to allow local production.

The PDA report discusses qualification and operational handling of passive thermal protection systems.