
A loan from the European Investment Bank will give BiondVax resources for Phase III trials and a manufacturing facility for its universal flu vaccine.

A loan from the European Investment Bank will give BiondVax resources for Phase III trials and a manufacturing facility for its universal flu vaccine.

The landmark decision determined that biosimilar makers can notify manufacturers before receiving FDA approval.

The company will expand its production facilities in Carlow and Cork to meet increased global demand for its medicines and vaccines produced in Ireland.
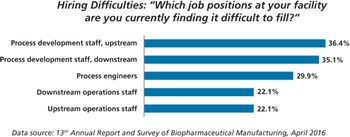
Contract manufacturing organizations may fill manufacturing gaps created by a lack of trained workers at Chinese biopharma companies.

Companies believe that biologics and biosimilars would experience the fastest growth over the next year; there is also interest on increasing market penetration of generic drugs.

The agency issued its recommendation for the influenza virus strains European vaccine manufacturers should include for 2017.

New study shows China biopharma companies face staffing shortages.

The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics

The industry is becoming more consolidated, but there needs to be some strategy behind the mergers and acquisitions.

The Generic Pharmaceutical Association announces a rebranding campaign to expand access to medicines.

Early-access schemes aim to make medicines available to patients faster but the regulatory framework remains unclear especially for biologics that involve complex manufacturing.

Merck will pay a one-time fee of $625 million and additional royalties to BMS and Ono Pharmaceutical to settle the patent infringement case related to Keytruda.

In a blog post, Robert Califf and Rita Nalubola discuss the agency’s approach to the use of genome-edited products.

The agency plans on publishing more than 100 new or revised guidance documents in 2017.

The partnership will focus on providing practical information to clients on the development of biologics and vaccines.
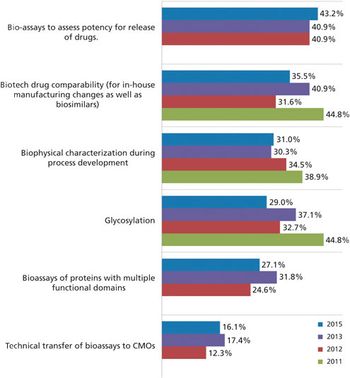
With a healthy supply of biosimilars heading to the approval pipeline, will license holders turn to contract manufacturers for development and production?

BMS changes its US geographic footprint with R&D investments and closures.

Biosimilars may be the key to CMO growth.

Takeda will invest more than 100 million Euros to build a new manufacturing plant for its dengue vaccine candidate in Singen, Germany.

Avecia is adding drug substance capacity at its Milford, MA manufacturing site.

German biotech ARTES and Iranian biopharma manufacturer BioSun will develop an HPV vaccine.

Astellas will acquire Ganymed and its portfolio of ideal monoclonal antibody candidates.

The Koolit Advanced PCM Gel from Cold Chain Technologies provides cold-temperature protection for drugs and vaccines during transport.

Regulators are tightening up on post-marketing monitoring of biological medicines to detect deficiencies caused by manufacturing problems, particularly those stemming from post-authorization changes in the manufacturing process.

Ravi Limaye gives an overview of the biosimilar industry and projects for 2020.