
Acasti Pharma and CDMO CordenPharma designed and implemented a continuous process for purifying raw krill oil for production of omega-3 phospholipid.

Acasti Pharma and CDMO CordenPharma designed and implemented a continuous process for purifying raw krill oil for production of omega-3 phospholipid.

The author shares his opinion on the challenges presented by the Internet of Things and what companies need to consider when choosing suitable architectures to manage serialization data.

Cambrex announces expansions of its North Carolina API pilot plant and Kalskoga, Sweden large-scale manufacturing facility.

The new UPS facility in Columbia will serve the growing pharmaceutical, biopharma, and medical device industry in Latin America.

As cost pressures mount as a result of multiple biologics being developed for the same indication, manufacturers can harness process efficiencies to maintain the value of legacy products.
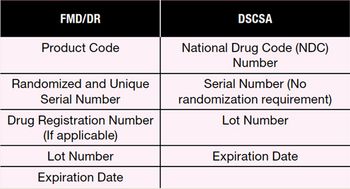
The author discusses upcoming serialization and transaction data collection regulations including the US Drug Supply Chain Security Act, the European Union Falsified Medicines Directive, and the EU Delegated Regulation.

Serialization and complementary authentication technologies are needed in order to meet DSCSA and FMD regulations.

The past several months have seen new product releases and updates made to already available laboratory equipment.

Modern air jet milling can be used to investigate the feasibility of micronization as a solubilization approach in formulation development.

Follow guidelines for E&L studies of an orally inhaled and nasal drug product formulation in its delivery device.

The company recalled the tablets due to a packaging error.
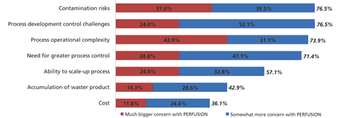
Although widespread adoption of continuous bioprocessing has been slow, some processes have been an exception.

Single-use systems demonstrate advantages over stainless-steel systems for biopharmaceutical manufacturing facilities.

BioVectra will open its new microbial fermentation and complex chemistry site, including the capability to handle high-potency APIs, by the end of 2017.

The pharmaceutical industry is making efforts by internally assessing, developing, and implementing semi-continuous manufacturing processes to improve manufacturing efficiencies.

The company will expand its production facilities in Carlow and Cork to meet increased global demand for its medicines and vaccines produced in Ireland.

The new storage and distribution facility provides PCI with additional space, complementing its existing footprint currently used for specialist clinical-trial logistics as well as packaging, labeling, and qualified person activities for investigational medicinal products.

A recent survey suggests that 36% of pharmaceutical companies and contract development and manufacturing companies have not started working on serialization, and that those who are working on it are focusing on basic compliance rather than potential long-term business benefits.

Due to weak penalties and lax enforcement, illicit pharmaceuticals continue to slip into the US, European and global supply chains.

Saneca has been granted approval to manufacture and supply multiple dosage forms to Russia.

Catalent has signed an agreement with Therachon to support preclinical and clinical development of TA-46, a novel protein being developed to treat achondroplasia.

Catalent has been working together with Lexicon to develop the drug formulation of Xermelo, which has now been approved by FDA for the treatment of carcinoid syndrome diarrhea.

Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

A multi-pronged approach to raw materials testing can help mitigate the risk of future contamination events.

Good project management, budgeting, planning, and clear documentation are the only ways to prevent overruns and project failure.