
Schreiner MediPharm’s multi-functional booklet-label combines several digital security features with an integrated first-opening seal.

Schreiner MediPharm’s multi-functional booklet-label combines several digital security features with an integrated first-opening seal.

QF20kSU single-use pumps from Quattroflow are used for applications requiring gentle product handling, high containment, low pulsation, purity, and cleanability.

Herma’s 152E Wraparound Bottle Labeler eases integration of print and vision systems.

Banner Engineering’s Sure Cross U-GAGE K50U Ultrasonic Sensor is used for wireless tank monitoring.

The patented SKAN NANOX Catalysts enable higher efficiency breakdown of hydrogen peroxide at lower temperatures for shorter decontamination cycles.

Parenteral packaging will be well-represented at INTERPHEX, especially technologies associated with fill/finish of ready-to-use vials, cartridges, and syringes.

The company made a €42 million investment in a new building at its Reinbek site to support the production of biopharmaceuticals.
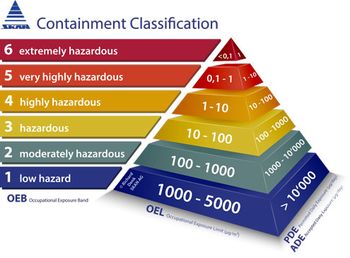
The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

Moving to the next level of productive, reliable performance in bio/pharmaceutical manufacturing requires a willingness to make changes and create a quality culture.
The DME Facility Focus survey revealed best practices for coping with the challenges of aging facilities and implementing facility modernization.

The Mutual Recognition Agreement will allow FDA and EU inspectors to recognize each other’s work and avoid the duplication of drug inspections.

Industry experts discuss IIoT and its impact on pharmaceutical manufacturing.

Pharmaceutical Technology spoke with Frank Generotzky, plant manager for Baxter BioPharma Solutions’ Halle, Germany facility, about operational excellence at the site.

Capsule filling is a complex process, and the product to be encapsulated must be well developed to ensure mass uniformity.

Although best practices are key, advances in integrated informatics platforms and automation can make it easier to ensure data integrity and improve overall lab efficiency.

As the November 2017 deadline nears, a surprising number of companies still don’t have a serialization plan in place. New programs aim to get them compliant in time.

The UK Stem Cell Bank released validated stem-cell lines for researchers developing novel cell-based therapies for clinical trials.

The article reviews strategies for firms with or without existing in-house capacities and the pros and cons for outsourcing bio/pharmaceutical development and manufacturing.

Having an effective and granular data management process in place will enable companies to meet the requirements of IDMP as well as help usher in a new age of digital-based identification, in which organizations can easily share data across borders.

The agency cited the company’s Kansas facility with CGMP violations similar to problems found at other Hospira facilities.

Avella issued a nationwide recall of sterile products produced at the Advanced Pharma Houston location due to inaccurate labeling.

Sanofi and Lonza formed a joint venture to build and operate a large-scale mammalian cell culture facility for monoclonal antibody production in Visp, Switzerland.

The company is voluntarily recalling one lot of Edex due to a lack of container closure integrity.

The industry is becoming more consolidated, but there needs to be some strategy behind the mergers and acquisitions.

The authors discuss regulatory and patent issues with combination products.