
Bosch’s Xelum platform is designed for continuous production of oral solid-dosage forms.

Bosch’s Xelum platform is designed for continuous production of oral solid-dosage forms.

The company is expanding their April 2017 voluntary recall of phenobarbital tablets.

FDA sent a warning letter to Pharmaceutic Labs, LLC for deficiencies in producing sterile drugs.

EMA and the European Commission released a biosimilars information guide for health professionals during the EC’s biosimilars conference.

Learn how to prevent common causes of product loss.

Robotic fill/finish systems reduce human intervention, improve flexibility, and allow more gentle handling of containers.

Recent legislation and PDUFA initiatives aim to streamline oversight and testing requirements.

Innovative new technologies released over the past several months seek to enhance bio/pharmaceutical development and manufacturing.

Advances in equipment, instrument, and control systems are enabling online monitoring of continuous API manufacturing.
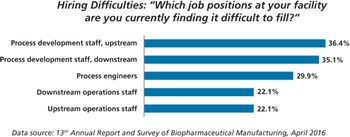
Contract manufacturing organizations may fill manufacturing gaps created by a lack of trained workers at Chinese biopharma companies.

Value-based medicine is putting patients at the center of pharmaceutical R&D and forcing the industry to move from treatment to prevention.
The author discusses the impact of prefilled syringe product contact materials and the filling and stoppering process on protein aggregates.
Transdermal and inhaled/nasal delivery provide alternative routes of administration for macromolecules.

Solid-phase extraction has several advantages over liquid/liquid extraction for extractables and leachables studies.

Avid, a wholly owned subsidiary of Peregrine Pharmaceuticals, will upgrade its Myford, California clinical and commercial manufacturing facility with multiple Mobius 2000-L single-use bioreactors from MilliporeSigma, the companies announced on May 1, 2017.

The type of water for pharmaceutical use is determined by USP testing.

Innovative new technologies released over the past several months seek to enhance bio/pharmaceutical development and manufacturing.

Companies believe that biologics and biosimilars would experience the fastest growth over the next year; there is also interest on increasing market penetration of generic drugs.

Sandoz, a Novartis division, announced that the Committee for Medicinal Products for Human Use (CHMP) has adopted positive opinions recommending the approval of its biosimilars rituximab and etanercept in Europe, for the same indications as the respective reference medicines.

The new suite will be used to produce non-sterile dosage forms, such as metered dose inhalers and semisolid topical products, for clinical studies up to Phase II.

The agency is warning about the potential threat of respiratory depression in children who take medicines with codeine or tramadol.

Although technical paths for continuous solid-dosage manufacturing have been laid out and equipment and control systems have been developed, industry is slow to move forward.

Pharmaceutical Technology spoke with Brad Pedrow and Rajesh Singh of Deloitte Consulting to discuss serialization implementation, and what to expect as the DSCSA deadline approaches.

igital tracking of overall equipment effectiveness can improve efficiency.

Cobra will increase capacity in response to customer demand for DNA and viral vector production.