
Onset expanded its product line for pharmaceutical cold-chain management with the launch two new products.

Onset expanded its product line for pharmaceutical cold-chain management with the launch two new products.

Sharp Packaging Services adds Biotechnology Center of Excellence to its to its Allentown, PA campus.

Wells Pharmacy Network is voluntarily recalling all of its products due to sterility concerns.

R-Pharm facility in Yaroslavl, Russia, is designed to produce biological drugs with GE Healthcare's FlexFactory manufacturing platform.

Researchers from the Wyss Institute explain a potential method for transporting and producing temperature-sensitive pharmaceuticals at a reduced cost.
Award-winning drug packaging innovations include interactive cartons and multifunctional labels.

Manufacturing data collected in the process historian can be analyzed to better understand the process and use past behavior to predict future results.

Genentech’s biologics drug substance plant is the overall winner of the International Society for Pharmaceutical Engineering’s 2016 FOYA Awards.

Clinical biotechnology company Moderna Therapeutics will build an integrated clinical manufacturing facility for mRNA production in Norwood, Massachusetts.

Mylan CEO Heather Bresch appeared before the House Committee on Oversight and Government Reform on Sept. 21, 2016 to explain the company’s decision to increase the price of EpiPen more than 400%. The meeting comes after multiple members of Congress raised concerns about the price of the life-saving drug, which is used to treat anaphylaxis.

The company sees a demand for customized individual packaging solutions as the pharmaceutical manufacturing industry moves towards smaller lot sizes that require aspects of individualization.

GE Healthcare’s GE BioPark Cork will hold four KUBio manufacturing facilities; GE will also collaborate with NIBRT for biopharmaceutical training.

Best practices for buying, selling, and transporting second-user equipment.

Industry concerns have generated efforts by FDA to streamline the system for designating the lead center to regulate a new combination product.

FDA sent a warning letter to an Illinois compounding pharmacy for violations of the Federal Food, Drug, and Cosmetic Act.

The Gx RTF ClearJect syringe is made of cyclic olefin copolymer and is tungsten- and adhesive-free.

The Novelia system has been approved as a packaging component and delivery system of multidose drug product formulated without preservatives.

Saneca Pharma is making significant investment in its API capabilities to support client demand for smaller batch sizes and streamlined scale-up.

The new regulations aim to introduce greater consistency and uniformity in the assessment and approval of medical devices and in-vitro diagnostics across the European Union.

If FDA’s proposed generic-drug labeling rule is passed in 2017, generic drug companies would need documented processes for safety tracking and label updates.

Mumbai, the location for its new Indian subsidiary, will also be the site for the company's Uniquity Global Conference on October 6, 2016.
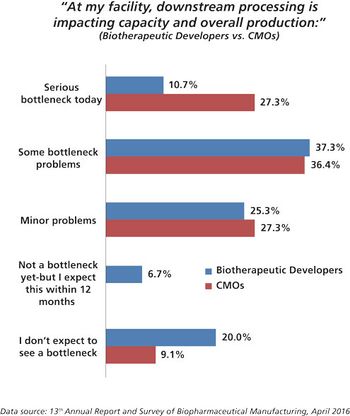
CMOs experiment with new filtration and purification methods to alleviate downstream bottlenecks and stay competitive.

In the pharmaceutical factory of the future, data collected by internet-connected manufacturing equipment improves operational efficiency.

Renovating a facility requires careful design and a plan to minimize production interruption.

Industry experts discuss recent trends in modular manufacturing.