
The authors of "Common Deficiencies in ANDAs for Dermatologic Drug Products" provide some examples of commonly cited deficiencies cited by FDA.

The authors of "Common Deficiencies in ANDAs for Dermatologic Drug Products" provide some examples of commonly cited deficiencies cited by FDA.

Flexible batch sizes for semi-continuous unit operations, such as tableting and encapsulation, can improve efficiency while maintaining quality.

Sound process understanding and having effective controls in place are crucial in ensuring that consistent product quality is obtained during API manufacturing.

One of the major challenges in working with excipients today is understanding, and adjusting to, complexity in materials and formulations.

Innovative technologies and services meet needs for existing and emerging biologic-based therapies.

The platform combines an expression system with equipment and process controls to enable rapid development and scale-up of robust titers.

CSafe completed the acquisition of Kallibox, a manufacturer of cold-chain packaging solutions.

GE and Sealed Air extend their long-term collaboration to develop single-use films purposefully constructed for bioprocessing applications.

The company announced that it will be expanding its portfolio of single-use vessels for fermentation.

Evonik’s parenteral drug delivery and commercial drug product manufacturing in Alabama passes EU GMP inspection.

FDA cites two Xinxiang Tuoxin Biochemical facilities with CGMP deviations.

The agreement comprises technology transfer and commercial manufacturing of Tillotts’ gastroenterology products.
Improvements to aseptic manufacturing procedures are long overdue. But how feasible is it for manufacturers to modernize fill lines of legacy products?

InstantGMP PRO 3.0 software integrates production scales with an electronic batch record system.
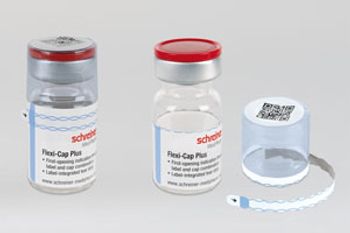
Schreiner MediPharm’s Flexi-Cap Plus incorporates a label-integrated tear strip for enhanced security.

A new version of Siemen’s Simatic EBR software offers web-based master batch records and integration with automation systems.

Dalton Pharma Services completed an expansion in sterile filling and API manufacturing at its cGMP facility in Toronto, Canada.

The company is voluntarily recalling one lot of product due to a potential packaging mistake.

Recipharm is investing EUR3.7 million (approximately $4.18 million) to increase lyophilization capacity at its Masate facility in Italy.

The company is opening two offices in the United States that will offer serialization, automation, and process control services.

Fresenius Kabi will add to its generic, sterile injectable manufacturing at its Melrose Park, Illinois site.

Arbor Pharmaceuticals is voluntarily recalling Cetylev (acetylcysteine) effervescent tablets due to inadequate seal of the blister pack.

TOPAS Advanced Polymers announces its COC materials are compliant with new USP standard for pharma plastic packaging systems.

The Group is focusing on standardizing data exchanges between the enterprise serialization management function and product packaging lines.

Piramal Enterprises has entered into an agreement to acquire 100% stake in Ash Stevens all by cash for a consideration of $42.95 million plus an earn-out consideration capped at $10 million.