
Merck will pay a one-time fee of $625 million and additional royalties to BMS and Ono Pharmaceutical to settle the patent infringement case related to Keytruda.

Merck will pay a one-time fee of $625 million and additional royalties to BMS and Ono Pharmaceutical to settle the patent infringement case related to Keytruda.

A new report says that failure to account for rebates, discounts, and price concessions leads to an “overstatement of payments realized by manufacturers” in most annual industry drug spend reports.

Camfil Air Pollution Control has expanded its testing laboratory and added a dust collection test rig for the ANSI dust collection standard.

The company will begin trials with its CBD chewing gum, CanChew Plus, for the treatment of irritable bowel syndrome.

The presentation details the addition of triethylamine to the guideline on toxic solvents.

In a blog post, Robert Califf and Rita Nalubola discuss the agency’s approach to the use of genome-edited products.

With this new line, Bischof + Klein will double its extrusion capacities for its CleanFlex clean room films.

The company opened a facility in Spain dedicated to the production of meglumine.

The agency plans on publishing more than 100 new or revised guidance documents in 2017.

B&W Tek’s director of Market and Customer Development, Katherine A. Bakeev, PhD, discusses analysis of pharmaceutical raw materials and the benefits of using handheld Raman spectrometers.

Language surrounding regenerative medicine and the REGROW Act appeared back into the 21st Century Cures Act right before it passed. What will this mean for the controversial testing and marketing of stem-cell therapies?

The partnership will focus on providing practical information to clients on the development of biologics and vaccines.

GEA’s ConsiGma continuous tableting line combined with Siemens’ automation and Sipat data management systems enables continuous manufacturing.

Catalent announces a development agreement with JOT to evaluate softgel options for a resveratrol drug candidate.

The company was cited by FDA for violations of sterile processing GMPs.
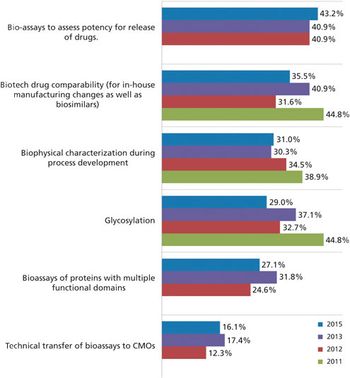
With a healthy supply of biosimilars heading to the approval pipeline, will license holders turn to contract manufacturers for development and production?

Prokarium in the UK and Probiomed in Mexico hope to demonstrate large-scale production of oral vaccines that will reduce cost compared to injections and improve availability.

TraceLink’s first Drug Supply, Safety and Traceability Report suggests that 19% of pharma companies, 11% of distributors, and 9% of dispensers still haven’t begun to address lot-level serialization requirements. Only 20% say their CMOs will be ready for the November 2017 deadline.

Pylote SA discusses its Pyclear Protection packaging innovation.

Moisture uptake during the end-to-end manufacturing process and supply chain can affect product quality. Simulation tools based on mechanistic models help define storage and handling requirements for oral solid-dosage drugs.

Advances in materials and equipment for pharmaceutical blister packaging protect quality and enhance shelf life.

Healthcare policies, R&D investments, and drug approvals will test bio/pharma.

The authors describe the development and validation of a highly sensitive point-of-use pressure decay test.

As pharmaceutical quality metrics evolve, they will need to incorporate more of the principles of operational excellence, says consultant Prabir Basu.

The co-located Pharma EXPO and PACK EXPO International presented technologies to protect products and people.