
The authors describe some cases of container closure design flaws and actions taken by FDA to mitigate safety risks and increase patient acceptability.

The authors describe some cases of container closure design flaws and actions taken by FDA to mitigate safety risks and increase patient acceptability.

WuXi AppTec’s new biomanufacturing facility is its third facility in the Philadelphia, PA Navy Yard.

A report from the European Union Intellectual Property Office shows that the EU loses approximately €10.2 billion a year due to counterfeit medicines.

GEA’s ConsiGma, which is a multipurpose continuous manufacturing platform, won the award for excellence in manufacturing technology and equipment at the 2016 CPhI Pharma Awards.

Pyclear Protection replaces the use of antimicrobial preservatives in multi-dose products without any change to the primary packaging and has no impact on the manufacturing process.

The Intelligent Control Inhaler is an intuitive, fully-integrated device delivering accurate doses of medication to patients, while providing on-screen instructions for use and feedback to the patient and healthcare provider via an app.

Novasep’s BioSC Lab chromatography is a flexible equipment that enables operations from one column up to six columns in batch, parallel batch, or continuous processing with all media and membrane types.

Pharma Venture’s approach in tackling supply-chain, logistic, and distribution challenges in the MENA region was recognized at the CPhI Pharma Awards.

The winning entry was Merck’s Emprove program, which facilitates risk assessment and supplier qualification by providing instant, online access to regulatory and technical information on hundreds of products used in pharmaceutical and biopharmaceutical manufacturing.

The time and resources required to finalize post-approval changes may be preventing manufacturers from modernizing facilities, or even scouting for new technology.

The Koolit Advanced PCM Gel from Cold Chain Technologies provides cold-temperature protection for drugs and vaccines during transport.

A PESU membrane is now available for Sartorius Stedim Biotech Sartocon benchtop and production-scale filtration assemblies.

Pharmaceutical manufacturers and business partners are studying how Advanced Digital Ledger Technology might solve supply chain and other data transfer problems.

PaizaBio will add aseptic injectable production capacity in Hangzhou, China.

This article will equip the excipient vendor with an understanding of QbD from the perspective of the topical pharmaceutical product manufacturer.
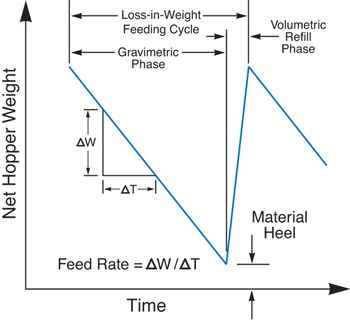
Designing loss-in-weight feeders for accurate and consistent refill is crucial to a continuous solid-dosage process.

Primary packaging and manufacturing technologies minimize product/package interaction, protect quality, support safe travel through the supply chain, and enhance performance at point of use.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the assessment of risk in the processing of intravenous injectable drugs.
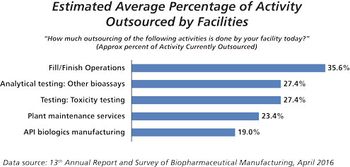
This key bioprocessing segment is expecting continued growth

The editors seek drug development experts to contribute technical articles for 2017.

Excipients play a crucial role in the manufacturing of solid-dosage forms and the performance of the finished drug product.

A number of organizations have analyzed and estimated the size of pharma’s counterfeit and diversion problem.

As criminals duplicate the latest overt security technologies, pharmaceutical manufacturers are evaluating covert and layered approaches to fight counterfeiting, theft, and illegal product diversion.

Researchers develop catalysts that mediate complex transformations under conditions appropriate for commercial manufacture.

Regulators are tightening up on post-marketing monitoring of biological medicines to detect deficiencies caused by manufacturing problems, particularly those stemming from post-authorization changes in the manufacturing process.