
The Ross Inline Solids/Liquid Injection Manifold (SLIM) Mixer is designed to work with a range of powders that are challenging to mix into liquids.

The Ross Inline Solids/Liquid Injection Manifold (SLIM) Mixer is designed to work with a range of powders that are challenging to mix into liquids.

Potential for improved product quality and cost/time savings is reviving interest in perfusion technology.

Create an efficient global labeling strategy that is compliant with both electronic and paper-based package-insert requirements.

Ignoring a contract partner’s ability to handle highly potent APIs (HPAPIs) safely may have serious consequences. Drug owners and contract service providers alike must understand the complexities and liabilities involved in working with HPAPIs.

Cloud computing has made it easier for pharma companies and their contract partners to gain visibility into their combined supply chains.
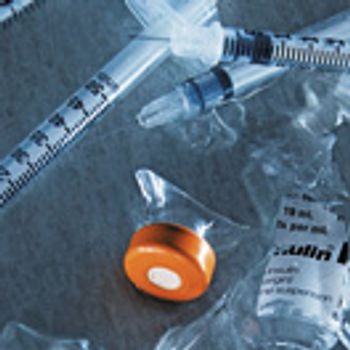
Within the past few years, key players have left the sterile manufacturing business. Can new technology and investment revitalize this critical market?

Sponsors and contract partners alike should not assume that upcoming US federal deadlines will be as elastic as California’s were.

The investment doubles the company’s capacity to meet increasing demand for its biologics contract testing and biosimilar characterization services.

Teva Parenteral Medicines initiates voluntary nationwide recall of select lots of Adrucil due to particulate matter.

Baxter has recalled of one lot of IV solution due to the potential for leaking containers, particulate matter, and missing port.

Lupin acquires businesses in Germany, the US, Russia, Brazil, and South Africa.

Avista grows its contract services business, and Scynexis focuses on antifungal development.

The contract is a memorandum of understanding for the manufacturing by MaSTherCell of clinical batches of TxCell’s Ovasave.

Manufacturers are producing new drugs and vaccines and clinical supplies faster and more efficiently through the development of standards and common practices for single-use technology systems.

SafeBridge Consultants reports on the status of nine companies in the Potent Compound Safety Certification Program.

Investment at Capsugel’s Edinburgh, Scotland facility will expand the company’s liquid- and semi-solid-fill capsule manufacturing capacity.

CSafe and AES will support controlled-temperature shipping through the Basel, Switzerland airport from their new service center.

Biogen will take ownership of Eisai's Research Triangle Park manufacturing campus and will manufacture both oral solid-dose and parenteral drugs for Eisai.

Baxter has initiated a voluntary recall of two lots of IV solutions due to the potential presence of particulate matter.

The EMA, FDA, and EC met to plan collaboration on pharmaceutical drug development and evaluation.

Sanofi's new business-unit structure focuses on growth drivers.

Improved characterization of twin-screw extrusion enables increasing use in pharmaceutical manufacturing.

Checkweighers, metal detectors, x-ray inspectors, leak detectors, headspace analyzers, and optical inspection systems for packaging were demonstrated at INTERPHEX 2015.

The new sizes follow the 2014 launch of the company’s fully disposable purifier.

The agency requires early notification of potential drug shortages.