
Herma’s 132M HC labelling machine is suitable for applications that require high-performance wrap-around labelling.

Herma’s 132M HC labelling machine is suitable for applications that require high-performance wrap-around labelling.

Products on display include the newest addition to the Mobius line of single-use bioreactors; Cellvento CHO cell culture media for optimized production of specific cell lines; and new high-volume AFS water purification systems.

Porvair has developed a 2-ml 96 round-well deep-well plate that enables scientists to use the full 2.00 ml well capacity.

GEA’s tableting technologies include a unique system that independently and simultaneously measures and controls both tablet weight and hardness, and a weight control system that provides increased sensitivity at lower forces.
![Image_1_PSL_Dispensary_Isolator[1].jpg](https://cdn.sanity.io/images/0vv8moc6/pharmtech/bf6523e0f089da35fe8d74a4afd23f9b90ee39f2-1000x831.jpg?w=350&fit=crop&auto=format)
PSL has developed a dispensary isolator with a contained drum hoist mechanism for highly potent API production.

This latest model is said to offer higher output and more efficient dedusting process.

GEA will showcase the ConsiGma continuous coater and the PCMM (portable, continuous, miniature, and modular) manufacturing technology platform for continuous production of oral solid dosage forms.

Telstar has developed an innovative in-line vial management system for recognition and marshaling of vials in automatic loading and unloading systems.

The Green Cross facility in Canada will produce intravenous immunoglobulin and albumin.

Machine manufacturer IMA is showcasing a number of technological advances for the processing and packaging of pharmaceutical products at ACHEMA 2015.

The Hapa 862, from Hapa AG, is a modular, CMYK/spot-color inkjet printing system for the in-line packaging printing of foils and labels.

Steve Osborn, product design manager at tablet tooling manufacturer I Holland, spoke to Pharmaceutical Technology Europe about the key considerations for successful implementation of multi-tip tooling in tableting operations.

FDA cites 17 observations including air handling, quality control, and deficient microbial monitoring at NIH’s Clinical Center Pharmaceutical Development Section.

Successful drug delivery via a dry powder inhaler is determined by the API physicochemical properties, the formulation composition and process, the device and operating conditions, the patient–device relationship, the environmental variables, and ultimately, patient compliance.
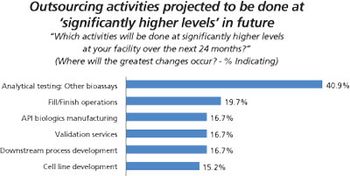
Biopharma companies on both sides of the Atlantic ship more of their assay testing to outside service providers.

Clearly defined zones of cleanliness must be designed and maintained to prevent product contamination.

Safer reagents and reaction conditions are making many hazardous transformations possible.
PAT holds the key to real-time quality assurance and consistent product quality in pharmaceutical manufacturing.

Pharmaceutical Technology spoke to experts in the field of biopharmaceutical manufacturing to gain insights on top trends that are currently shaping the industry.

Careful choice of wash-water parameters and attention to water quality and basket loading are important for optimal cleaning.

Hosokawa Micron’s Mini Cyclomix lab mixer is designed to blend formulations for dry-powder inhalers without deteriorating the particles.

Ross’ Multi-Shaft Mixers with interchangeable mixing vessels include an optional cover featuring propeller blades.

Thermo Scientific’s Spinnaker Smart Laboratory Robot, featuring Thermo Scientific Momentum 4 software, is designed to eliminate the need for manual correction of drift by automatically compensating for positional variations.
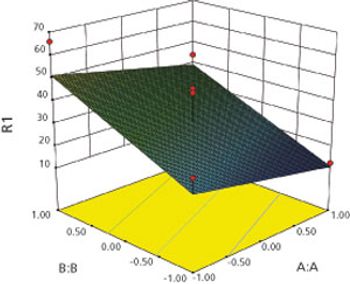
Box-Behnken modeling was used to optimize a resinate complex, to mask the taste of levocetirizine dihydrochloride and montelukast sodium in orally disintegrating tablets.

ProMetic Life Sciences will use manufacturing and plasma fractionation processes at Emergent BioSolutions’ Winnipeg, Canada facility.