
Biogen plans to build a biologics manufacturing plant in northwest Switzerland using next-generation technologies to create efficiency and sustainability.

Biogen plans to build a biologics manufacturing plant in northwest Switzerland using next-generation technologies to create efficiency and sustainability.

Electronic pharmaceutical tablet counters meet demands for accuracy, flexibility, speed, compact size, easy cleanability, and quick changeover.
Biologics exhibit greater variability in stability testing than do small-molecule drugs, and maintaining a stable test environment is crucial.
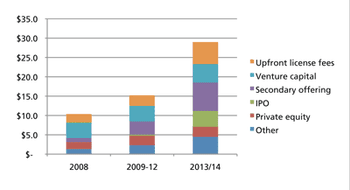
While all market signs are pointing up, memories of past setbacks may discourage from expanding capacity.

PTI Inspection System’s VeriPac 310 leak detection and package integrity testing system reduces waste and provides the user with a clear evaluation of package integrity.

Ross’ Double Planetary Mixer lined with Teflon is designed for high-purity applications and features resistance to chemicals and corrosive materials when stainless steel is not compatible.

The addition of a new manufacturing line at Lonza’s Portsmouth, NH site enables Alexion to add dedicated product supply for 10 years.

The new facility expands the company’s commercial manufacturing capability at its Bend, Ore. site.

Gil Roth, Founder and President of the Pharma & Biopharma Outsourcing Association speaks with Pharmaceutical Technology.

Richard Johnson, President and CEO of the Parenteral Drug Association (PDA) speaks with Pharmaceutical Technology.

Jim Miller, President of PharmSource, speaks with Pharmaceutical Technology.

Sue Schniepp, Chair, PDA Regulatory and Quality Advisory Board, and Hal Basemen, Chair, PDA, spoke with Pharmaceutical Technology about resolving drug shortages.

Jim Miller, President of PharmSource, spoke with Pharmaceutical Technology about working with CMOs and CDMOs.

Sue Schniepp, Chair, PDA Regulatory and Quality Advisory Board, and Hal Basemen, Chair, PDA, speak with Pharmaceutical Technology.

Eric Langer, Managing partner at BioPlan Associates, speaks with Pharmaceutical Technology about the results from the 12th annual BioPlan Associates survey, and single use technology adoption.

Eric Langer, Managing partner at BioPlan Associates, speaks with Pharmaceutical Technology about biosimilar development trends.

Bill Hartzel, Director Strategic Execution, Advanced Delivery Technologies at Catalent Pharma Solutions, spoke with Pharmaceutical Technology about blow-fill-seal for aseptic processes.

Brady Cole, VP of Commercial Operations, at ABEC, spoke with Pharmaceutical Technology about biopharmaceutical manufacturing processes.

Gordon Haines, Chief Executive Officer, and Kay Thiele, Head of Product Optimization, at Rottendorf Pharmaceuticals, spoke with Pharmaceutical Technology about the human element in pharmaceutical manufacturing.

Barry Holtz, Principal, at Holtz Biopharma Consulting, and Klyo Collaborative, spoke with Pharmaceutical Technology about collaborative success strategies for biopharm companies.

The agency gives a limited reprieve to dispensers but requires other trading partners to provide product tracing information.

GSK will invest in an additional downstream isolation facility for amoxicillin production in Singapore.

The new column features Natrix’s signature macroporous hydrogel.

The 12th Annual Report and Survey of Biomanufacturing is now available.
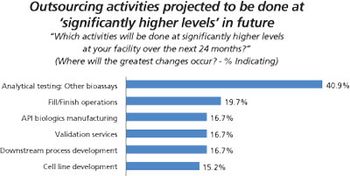
More biopharma companies choose outside service providers for assay testing.