
Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

According to AstraZeneca, the purchase of the biologics bulk plant will double the company's biologics manufacturing capacity in the US.

SGS Life Science Services announced that its facility in Fairfield, NJ, has been upgraded to be Biosafety Level 2 (BSL-2) compliant, according to the Centers for Disease Control and Prevention (CDC) guidelines.

The Krämer KCP10 upward-conveying capsule polisher elevates, polishes, and dedusts capsules.

Aragen Bioscience has licensed ProteoNic Biotechnology’s 2G UNic recombinant protein production technology, which increases manufacturing efficiency and reduces cost of goods for recombinant biologicals.

FDA warns that compounded or repackaged drugs stored in certain syringes made by Becton-Dickinson may lose potency because of an interaction with the rubber stopper.

This agreement is a follow-on to an established collaboration between LobSor and Recipharm for the development and the manufacturing of Lecigon batches for the recently completed clinical trial.

As EU supplier risk assessment deadlines approach, a number of voluntary third-party auditing and certification options are available. Will more excipient suppliers, and drug manufacturers, use them?

Creating closed processes and reducing room air classification in a biopharmaceutical facility can reduce operational costs.

Operator attention to detail and adherence to procedures are crucial for proper cleaning.

Current guidance for absorption of elemental impurities does not address dermal exposure, resulting in a simplistic approach to limit setting.
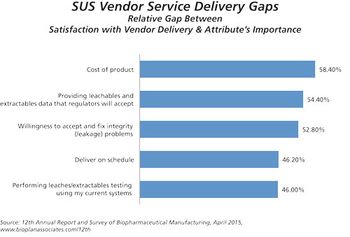
Suppliers indicate prices for single-use equipment are likely to increase.

The industry is moving towards more flexible manufacturing with the use of modular facilities and single-use systems.

Industry Expert Q&A with Robin M. Silva, Partner, Morgan, Lewis, and Bockius LLP

Adents’ Pharma Suite serialization software features track-and-trace capabilities.

Kason’s Vibro-Bed fluid-bed agglomerator is equipped with imbalanced-weight gyratory motors and mounted on a spring suspension.
Meissner’s FlexGro single-use biocontainer assemblies feature the TepoFlex polyethylene (PE) multi-layer film and are delivered presterilized for immediate use.

Ross’ Three Roll Mill features hardened carbon-steel 52100 precision ground rolls, each cored for water cooling and heating.

QbD is improving the safety of solid-dosage drug products as well improving manufacturing processes, despite some industry reluctance.
Process analytical technology is crucial for understanding a pharmaceutical or biopharmaceutical process. Thorough process knowledge is needed to develop process control strategies and select process equipment configuration for continuous manufacturing.

UPS joins the Global Health Supply Chain Technical Assistance program to help secure the drug supply chain.

To enable efficient monitoring systems, life-science companies need to effectively apply run rules.

The Pre-Connect Congress will explore pharma industry trends, such as mergers and acquisitions, the biologics market outlook, and innovation in drug delivery among others.

Growth is said to be driven by the deeper penetration of biosimilars in developed and emerging markets as a result of clearer regulatory pathways.

Novo Nordisk plans to invest approximately $2 billion over the next five years in new production facilities in Clayton, North Carolina, US and Måløv, Denmark. The facilities are expected to be operational during 2020.