
The Cell History File is designed as a “cell passport” for developers and manufacturers of tissue- and cell-based medicinal products.

The Cell History File is designed as a “cell passport” for developers and manufacturers of tissue- and cell-based medicinal products.

Probiodrug has signed an agreement with Rentschler Biotechnologie GmbH for the development of PBD-C06, a pGlu-Abeta-specific monoclonal antibody, as treatment for patients with Alzheimer’s disease.

The challenge of achieving zero visible defects (i.e., particulates) in parenteral drugs will require a coordinated effort at all stages of the supply chain, particularly in the production and filling of primary containers.

The PDA report discusses qualification and operational handling of passive thermal protection systems.
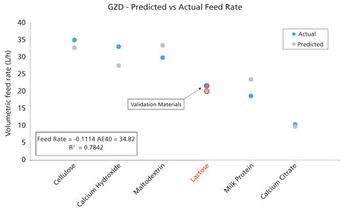
Multi-faceted powder characterization, including measurement of dynamic properties, can be used to correlate powder properties and process performance to support equipment selection, optimization, and troubleshooting.


Manufacturers of parenteral drugs face challenges to increase efficiency, control particulates, control extractables and leachables, and eliminate product/package interactions; new containers and packaging equipment offer increased options.

The complexity of new packaging regulations laid out in the Falsified Medicines Directive could threaten the existence of smaller pharma and packaging companies.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss performing investigations of biological products.

FDA selected USDM Life Sciences, RC Partners, and The Clarion Group to develop and implement a DSCSA pilot project.
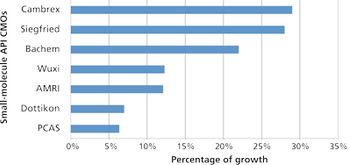
Despite emergence of biologics, small-molecule APIs benefit from industry growth.

The Alpha modular machine platform from Netzsch is designed to mount different grinding systems on the same base platform as appropriate for a defined drive capacity.

GEA’s Pony NS2006L is a self-contained, high-pressure laboratory and pilot plant homogenizer for product development of advanced fluid applications.

The flexible Dolomite Flow Chemistry Systems from Dolomite Microfluidics can be configured to suit different applications.
An integrated approach can improve the efficiency of cleaning validation studies.

Vetter plans to invest approximately 300 million Euros during the course of five years to expand drug product manufacturing and logistic services in Germany; upgrades will include an improved RABS system for aseptic processing.

CPhI’s Annual Report discusses the implications of QbD, continuous processing, excipient criticality, and process validation on pharmaceutical manufacturing and predicts a steady shift to continuous manufacturing.

Hovione will double its manufacturing capacity in New Jersey with an expanded facility and a commercial spray dryer designed to handle potent APIs.

The US Pharmacopeial Convention preposts its new chapter on sterile preparations for compounding pharmacies for public review.

There is growing interest in the development of liquid formulations in prefilled syringes and autoinjectors, which offer convenience and ease of administration in a home setting.

Cobra Biologics and the University of Manchester announce a collaboration to improve industrial scale-up of mammalian cell bioprocessing.

In testifying before Congress on FDA regulation of long-awaited biosimilars, Janet Woodcock emphasized the importance of ensuring that the evaluation of new therapies is based on sound science.

Novo Nordisk will build a local manufacturing plant for FlexPen prefilled insulin delivery devices in Iran.

ReposiTrak signs agreement to pilot track and trace system in pharma.

IMA Active’s new tablet press machine, Prexima, is designed to deliver higher productivity and efficiency.