
McNeil-PPC pleads guilty in connection with adulterated infants' and children's over-the-counter liquid medications.

McNeil-PPC pleads guilty in connection with adulterated infants' and children's over-the-counter liquid medications.

BIO and the Colorado BioScience Association urge Colorado Governor Hickenlooper to sign a bill that will help patients gain access to interchangeable biologic products following FDA approvals.

In a landmark decision, FDA approved Zarxio, making it the first biosimilar product in the United States.

BASF is expanding its Verbund site in Ludwigshafen to include production capacity for about 20 specialty amines.

Teva announced that it would sell its Sellersville, Pennsylvania facility to G&W Laboratories.

WellSpring Pharma Services has announced the appointment of David Mayers as president.

A facility expansion adds space for production of Repligen’s tangential flow system.

SGS Life Science Services adds analytical methods to identify amino acid impurities in bio/pharmaceutical manufacturing at facility in Germany.

Biosimilars may add a nice increment to the pipeline opportunities, for CMOs, but they are unlikely to be a bonanza for the industry.

New rules for tracing drugs through the supply chain and policies for drug compounders change the regulatory landscape.

The upgrades will offer the opportunity for higher product yields and higher purity levels.

Scientists and industry experts seek effective preventive therapies to combat global disease.

The fast growth of the global biopharmaceutical market has prompted global pharmaceutical and biotechnology companies to increase their R&D investment in biologics.
While the optimization of a lyophilization cycle for a biologic relies on a well-characterized formulation, viscosity and aggregation after product reconstitution must also be carefully managed.

Sessions address cell therapies, tableting, continuous processes, serialization, and more.

Assessing risk factors is key to implementing the new ICH Q3D guidelines for elemental impurities.

Cleanability is crucial when choosing components for GMP manufacturing areas.
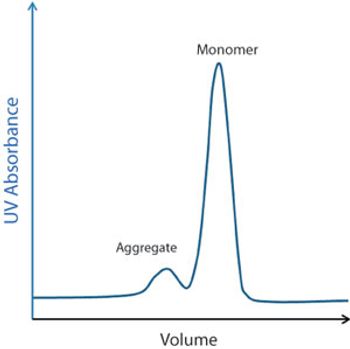
Several chromatographic resins are available for downstream purification.
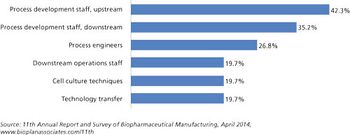
Is there enough talent to go around?

It is vital that companies involved in the manufacturing and handling of cytotoxic drugs ensure that staff are given the highest possible levels of protection.

The company has increased capacity for cold storage and controlled drug substances handling at its European facilities.

Enclosures contain powders and particulates during hazardous drug manipulation.

The company voluntarily recalls product due to FDA observations of potential sterility problems.

Aprecia Pharmaceuticals' new facility in Ohio will create 150 jobs.

Leased facilities in California will expand Kite Pharma's capacity for clinical T-cell therapies.