
Drug makers back alternative to FDA labeling update rule.

Drug makers back alternative to FDA labeling update rule.

Small- to medium-sized companies are expected to drive near-term growth of the global biomanufacturing outsourcing market.

Temperature-controlled packaging trends include prequalified systems that simplify adoption, reusable systems that are more sustainable, and new temperature-monitoring technology.

Biosimilar applicants will not be required to hand over their biosimilar applications and manufacturing dossiers to innovator companies, determines FDA.

FDA has concluded that Kemwell’s facility, systems, and practices comply with FDA’s requirements and no observations were reported on Form 483.

Genentech plans to invest more than $125 million in an expansion of its fill/finish facility in Hillsboro, OR.

FDA has approved another indication for Eylea, a treatment for diabetic retinopathy in patients with diabetic macular edema.

Use of modeling software can help improve process understanding, and can be used in open- or closed-loop control.

Emergent BioSolutions announced FDA approval of Anthrasil, an inhalable treatment that targets Anthrax toxins, as well as a contract with BARDA to develop NuThrax, an anthrax vaccine candidate.

The agency provides guidance on determining the need for environmental assessments for gene therapies, vectored vaccines, and viral and microbial products.

Guidance provides labeling standards and an implementation guide for electronic submission of lot distribution reports.

The BallProbe from MarqMetrix is an optical sampling interface for inline, real-time measurements.

Recipharm expects to be rolling out further serialization projects during 2015.

The investments made in analytical and process development equipment will enable Laurus Synthesis to offer chemistry services to its clients.

UPS expands its special commodities program to more countries.

Real-time, noncontact imaging and spectroscopy techniques provide insight into pharmaceutical processes.

A new machine is designed for inspection of prefilled syringes.
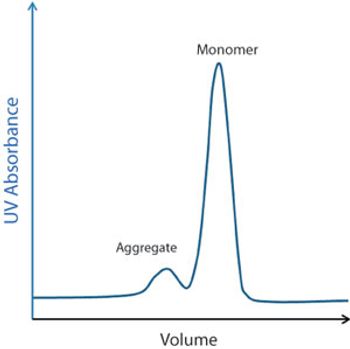
Several chromatographic resins are available for downstream purification.

Member of parliament, Desmond Swayne, will officially open the 42,000-square-foot facility on March 20, 2015.

The new flow sensors, BioPAT Flow, enable precise, reproducible data to be collected on flow rates and mass balances without coming in contact with the medium during measurement.

The manufacture and scale up production mark a significant milestone in the commercialization route of Nanobiotix lead product, NBTXR3.

WuXi's new facility in Philadelphia will manufacture CAR T-cell therapies and other cancer immunotherapies.

NSF's consensus-based standard incorporates multiple regulatory and industry requirements into a single rigorous standard for the manufacturing and distribution of pharmaceutical excipients.

Penn Pharma's equipment addition meets growing need for continuous dry granulation and for handling of highly potent drugs.

Innovative equipment, analytical techniques, software, and modeling systems are improving the tableting process.