
Serialization experts will share best practices and practical applications at INTERPHEX 2015.

Serialization experts will share best practices and practical applications at INTERPHEX 2015.

Catalent completes a $52 million expansion program for advanced oral solid manufacturing solutions at its Winchester, KY, facility.

PTI's next-generation VeriPac Inspection System, the VeriPac 310, uses new vacuum-decay technology to increase leak-detection accuracy for a range of flexible and rigid packages and containers.

Rentschler Biotechnologie expands European manufacturing capabilities with GE Healthcare Life Sciences bioprocess technologies.

A core coating module allows Roeltgen's FlexiTab development and small-batch tablet press to manufacture multi-layer and externally lubricated tablets.

India may go to the World Trade Organization if the EU does not reconsider its decision to suspend the sale of about 700 generic drugs that were approved based on clinical trials by GVK Biosciences.

Innovations include multimedia packaging and improved filling systems.

Experts discuss some of the latest trends in buying and selling used pharmaceutical equipment assets.

Schreiner MediPharm's low-migration labels for plastic containers use qualified adhesive systems, materials, and inks.

The single-use clarification system eliminates centrigues; harvesting can be performed in one step; and process robustness and predictability are ensured.

3C! Packaging's Clear Code coating enhances laser-etching on pharmaceutical packaging.

FDA issues a Warning Letter to Hospira S.p.A. for GMP violations at the company’s Liscate, Italy facility.

Baxter voluntarily recalls select lots of IV solutions due to possible particulate matter.

Flow indicators maximize the view into sterile processes.

Bosch Packaging Technology's RAN 3080 exterior washing machine removes product residue and other contamination from filled and closed glass containers using a new, efficient, high-pressure cleaning process.

Innovations for tablet tooling and presses improve quality and productivity.

Facilities in China, Ireland, Germany, and the United States have been recognized by ISPE in the 2015 Facility of the Year Awards program.

Pall’s acquisition of BioSMB from Tarpon Biosystems expands its downstream continuous processing offerings.

Catalent acquires Pharmapak Technologies, a pharmaceutical packaging company based in New South Wales, Australia.

GlaxoSmithKline announces global vaccines research and design facility to be based in Rockville, MD, USA.

Industry awaits the final revision of USP General Chapter and the impact it will have on the evaluation of sterile product package integrity.
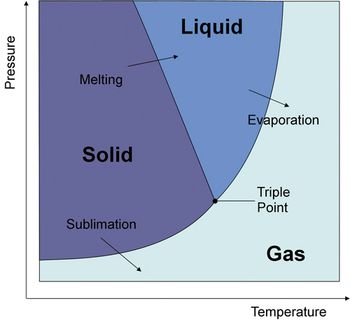
Efficient freeze-drying processes result in time and energy savings, reduced failure rates, and improved batch consistency.

Quality-by-design tools improve efficiency in scale-up of pharmaceutical processes.
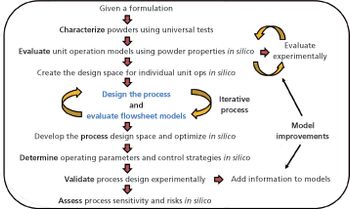
In-silico design facilitates process optimization and evaluation of process control strategies.

A drug-product manufacturing classification system (MCS) for oral solid-dosage forms is proposed by an Academy of Pharmaceutical Sciences working group.