
Pre-sterilized, nested syringes and vials are seeing increased use in sterile filling.

Pre-sterilized, nested syringes and vials are seeing increased use in sterile filling.

Comar's 18-mm SecureCap child-resistant closure for eye drops and nasal sprays meets US Consumer Product Safety Commission for products containing 0.08 milligrams or more of imidazolines.

Nelson Patterson has been elected to Pharma & Biopharma Outsourcing Association board. The Pharma & Biopharma Outsourcing Association (PBOA) has announced that Nelson Patterson, vice-president, sales and marketing at Baxter BioPharma Solutions, has been elected to its Board of Trustees, effective immediately. Patterson was elected after Baxter BioPharma Solutions joined the PBOA as a Sustaining Member.

Catalent announced that it would partner with Mitsubishi Gas Chemical Company, and its subsidiary MGC Pharma, to promote GPEx technology, a high-titer vector for stable mammalian cell lines.

Child-resistant packaging requirements for products containing a specified imidazoline will come into effect June 10, 2015.

Single-use components aid efficiency in automated personalized therapy manufacturing.

A guidance document published just before the Jan. 1, 2015 deadline adds a four-month grace period.

The acquisition of Aptuit's Glasgow, UK and Indiana, US facilities will add sterile injectable formulation development and expand AMRI's analytical services capabilities.

An FDA expert committee has recommend approval of Zarizio, the first US biosimilar application from Sandoz, setting a milestone for generic biologic drugs and setting the stage for future approvals.


The Parenteral Drug Association report addresses prevention and communication of drug shortages caused by manufacturing and quality related disruptions.
New materials and software modeling streamline the blister-package design process.

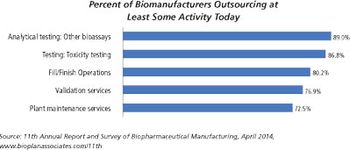
There are significant differences between small molecules and biologics fill/finish capacity.

Protein Sciences will evaluate sourcing Flublok from its Japanese licensee, UMN Pharma, which already runs a large-scale facility for the vaccine.

Bayer has appointed Cardinal Health as US-based contract manufacturer for Xofigo (radium Ra 223 dichloride) injection, and Cardinal Health will build a dedicated facility.

FDA has scheduled a public meeting in early January to assess and weigh data on the first United States application for a biosimilar therapy.

A global framework of standards enabling collaboration among industry stakeholders is needed to battle the increasing threat of counterfeit medicines.

New pharmaceutical packaging equipment includes advances in automation and efficient systems.

FDA funded projects at Rutgers' C-SOPS and at UMass Lowell to develop flow-sheet model algorithms for use in risk assessment, and start-up company Continuus Pharmaceuticals is making progress on their integrated system.

FDA drug approvals are up in 2014 with biologics drugs representing more than 25% of FDA approvals to date.

The multi-product biopharmaceutical manufacturing facility is scheduled to start up in 2017.

Biomanufacturing capacity expansion uses modular cleanroom design and single-use technologies.

Baxter announced that it had entered into an agreement to sell its Vero cell technology and related assets to Nanotherapeutics.

A new release of FactoryTalk Batch software from Rockwell Automation eases recipe creation and adds security.