
SAFC, Regis Technologies, Carbogen Amcis, Cambridge Major Laboratories, and AMRI are among the companies at CPhI Worldwide in Frankfurt this week that are moving forward with expansions.

SAFC, Regis Technologies, Carbogen Amcis, Cambridge Major Laboratories, and AMRI are among the companies at CPhI Worldwide in Frankfurt this week that are moving forward with expansions.

In the popular view of nanomedicine, miniature robots equipped with a set of tools will one day patrol the inside of the body, cleaning up atheroslerotic plaque, zapping cancer cells, and generally performing repair and maintenance.

The biggest benefit of PAT and the current FDA initiatives may be in allowing pharmaceutical scientists to use their technical capabilities to improve pharmaceutical processes.

Ionic liquids provide an alternative to certain solvents in select reactions used to synthesize intermediates and active pharmaceutical ingredients.

The days of a 'job for life' at a Big Pharma company seem to be well behind us, whether we're at the bench or in the boardroom, but that doesn't mean we should view the employment landscape with trepidation.

Nanoparticles can also cross the blood–brain barrier, which could make them useful for delivering drugs that target brain tumours or diseases that affect the central nervous system.

The potent nature of HPAPIs means there must be careful evaluation of the compound for its level of toxicity when considering manufacture.
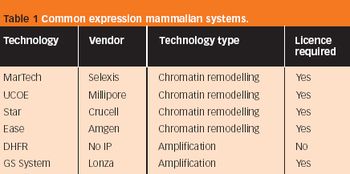
...an uninformed decision based on commercial requirements alone may, ultimately, have disastrous consequences on the efficacy of the target recombinants.

Also, Zentiva accepts Sanofi's increased takeover bid, Oriel Therapeutics appoints Richard Fuller CEO, more...

In a Sept. 17 letter to FDA Commissioner Andrew C. von Eschenbach, Rep. Henry Waxman (D-CA) questions the agency's priorities, specifically poking at FDA's political appointees and whether they are promoting industry at the expense of the public's health.

On November 3, the US Supreme Court will hear the case of Wyeth v. Levine, in which the drug company has challenged the ruling of a Vermont Supreme Court decision to award Diana Levine $6.8 million.

A new study concludes that an approval pathway for affordable follow-on biologics should be based on the Hatch–Waxman Act of 1984.

In an effort to clarify its policy on the use and creation of genetically engineered animals (GE animals), the US Food and Drug Administration released the draft guidance "The Regulation of Genetically Engineered Animals Containing Heritable rDNA Constructs" on September 18.

The US Food and Drug Administration has issued two warning letters to Ranbaxy Laboratories.

Also, Quintiles to expand Singapore operations; Christine A. Poon, chairman of Johnson & Johnson's pharmaceuticals group, to retire; more...

Earlier this month, the US Food and Drug Administration announced that it will be posting quarterly reports on its website regarding potential drug safety issues.

MannKind and Pfizer (New York) agreed that the former company will help patients who need inhaled insulin switch from Pfizer's "Exubera" medicine to MannKind's "Technosphere Insulin" drug.

The US Food and Drug Administration launched a new website to educate the general public about direct-to-consumer (DTC) advertising of prescription medications.

The US Food and Drug Administration has updated its draft bioequivalence recommendations for several products, and added 66 new draft product-specific guidelines since October 2007.

FDA has issued a Final Rule titled "Amendments to the Current Good Manufacturing Practice Regulations for Finished Pharmaceuticals."

Also, Human Genome Sciences enters pact with Hospira, Zosano Pharma names Gail Schulze chair and CEO, more...

Companies launched 25 new active substances in 2007, a decline of 19% from the previous year, according to a new report from Parexel International.

Also, Novartis stops development on "Aurograb," Zealand Pharma appoints David H. Solomon as CEO, more...

CMC service providers are doing well, but clinical and preclinical CROs are doing even better.

The US Food and Drug Administration will hold a public meeting this month to gain public input on implementing the recommendations of the Nanotechnology Task Force report, taking another step closer to setting a regulatory framework for nanotechnology for pharmaceuticals and medical devices.