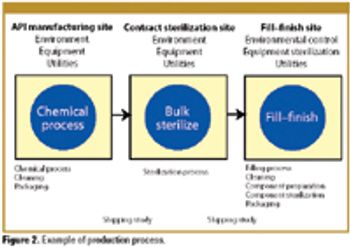
Patient safety must be the primary concern of any validation effort. The author explains how a risk-based approach to validation and compliance follows naturally from this premise.

Patient safety must be the primary concern of any validation effort. The author explains how a risk-based approach to validation and compliance follows naturally from this premise.
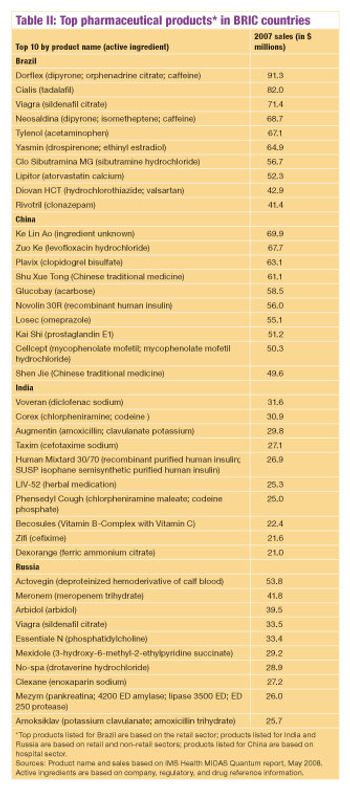
Recent reports discuss the future of pharma's emerging markets.

Editors' Picks of Pharmaceutical Science & Technology Innovations
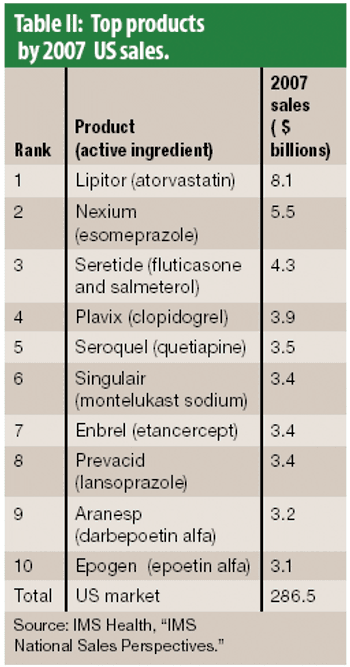
The year 2007 was slow for approvals for new molecular entities and overall pharmaceutical industry growth. Big Pharma seeks relief in a growing biologics portfolio.
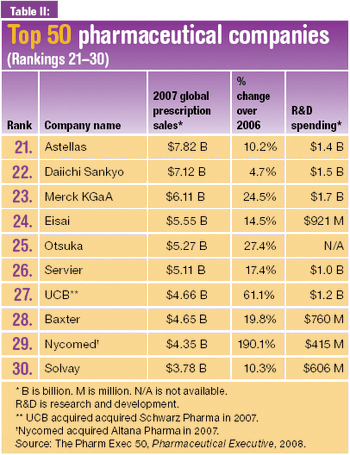
The pharmaceutical majors invest in biologics production capacity as they advance restructuring programs and build their pipelines

Last year, the Royal Pharmaceutical Society of Great Britain held a consultation regarding how the profession should develop during the next 12 years. An analysis of the results has just been published.1 It comes as no surprise to anyone who teaches pharmaceutics at UK pharmacy departments that one popular suggestion was to change the indicative syllabus so that it would focus more on clinical topics as opposed to scientific ones.

At first glance, the title of this article may bring a wry smile to the face of many an astute practitioner, but I can provide 'documentary evidence' that free validation is not a just a play on words, but a financial reality.

How can any company be sure that the standards that suppliers might claim to operate, and might be able to demonstrate from time to time, are actually being practised all the time?
Hood, suit, faceplate, cover shoes, gloves: these are the necessary items of clothing when operating in A-and B-grade areas.

... profit margins in ophthalmology are very attractive and the market is increasing as the incidence of eye disease grows with the ageing population.

The US Pharmacopeia (USP) posted revised monographs for heparin sodium and heparin calcium on its website.

Also, Stiefel Laboratories will acquire Barrier Therapeutics, Danube Pharmaceuticals appoints Brian Levy COO, more...

Bayer and its subsidiary Icon Genetics (Halle, Germany) have developed a production process to produce therapeutic proteins from tobacco plants.

Also, Patheon to expand Puerto Rico facility, Creabilis Therapeutics appoints Tony Wilson CEO, more...

Effective July 1, 2008, the Office of Generic Drugs (OGD) will require abbreviated new drug applications (ANDAs) to include data that demonstrate the manufacturer's control of residual solvents.

To choose the appropriate conveying system, a pharmaceutical manufacturer needs a thorough knowledge of the benefits and limitations of each conveyor type. The following overview compares several types of systems.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the June 2008 edition: Esco Technologies and Hawk Measurement

Manufacturers have stopped seeing disposable containment as an ad hoc solution, and the technology increasingly incorporates thoughtful design and engineering. The future promises to bring more changes and new applications for disposable containment technology.

In an assessment report about medicinal products containing heparin, the European Medicines Agency (EMEA)'s Committee for Medicinal Products for Human Use (CHMP) said it could not draw firm conclusions about the level of risk associated with unfractionated heparins (UFH) contaminated with oversulfated chondroitin sulfate (OSCS). Nevertheless, CHMP recommended that contaminated lots be withdrawn completely.

Also, Shire voluntarily recalls ADHD patch Daytrana

The steering committee of the International Conference on Harmonization and its expert working groups adopted Quality Guideline Q10 "Quality Systems" last week at a meeting in Portland, Oregon.

Also, Pall plans expansion in South America, Anthony Clarke joins Alexza Pharmaceuticals, more...

The US Food and Drug Administration issued a new guidance Monday on indexing structured product labeling. The Center for Biologics Evaluation and Research and the Center for Drug Evaluation and Research will begin indexing structured product labeling in the product labeling for human drug and biologic products.

The transatlantic cooperation of the European Commission, the European Medicines Agency, and the US Food and Drug Administration was recognized at the Second Meeting of the Transatlantic Economic Council, held mid May in Brussels. The TEC is tasked with overseeing and accelerating government-to-government cooperation to advance economic integration between the United States and the European Union.

The California Senate passed SB 1096 on May 29, voting to amend the state?s Confidentiality of Medical Information Act to allow pharmacies to provide third parties with patient information for the purpose of mailing prescription refill reminders and drug information directly to patients.