
Brief pharmaceutical news items for September 2008.

Brief pharmaceutical news items for September 2008.

This year, the employment survey will acknowledge the industry's best employers.

Identifying the most sustainable packaging for a product is rarely a simple exercise.

FDA is taking several measures to ensure that imported drugs meet manufacturing standards.
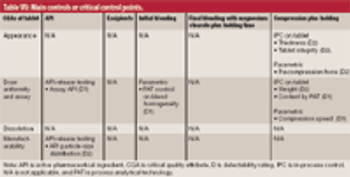
Criticality management combines pharmaceutical product, process, and material knowledge and risk management in one approach, which is reflected in a single document.

With economics and politics in the way, can we defeat the malaria epidemic before it defeats us?
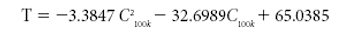
This tutorial paper is meant to aid in dielectric-sensor selection

Fueled by a need to reduce costs and improve efficiencies, continuous processing may be the next paradigm shift in pharmaceutical manufacturing.
Editors' Picks of Pharmaceutical Science & Technology Innovations

Operators are hit hard by breakage problems and strike out on FDA inspections.

Are hypersanitation trends a result of scaremongering or a lack of faith in medicine?

Researchers at Children's Hospital Boston (MA, USA) recently announced another group of stem cells that can produce cardiomyocytes (heart muscle cells). It seems hardly a month goes by without some kind of discovery in the fast-moving world of stem cells.

...some companies complained that what they received was not joint advice or combined advice but parallel advice, without coherence...

Pharmaceutical Technology Europe interviews Bruce Davis, Chairman of the International Society for Pharmaceutical Engineering Board (ISPE), the world's largest not-for-profit association dedicated to educating and advancing pharmaceutical manufacturing professionals and their industry.

We have to make the politicians understand that if we don't take biotech seriously then tourism will be our premier industry in the future.
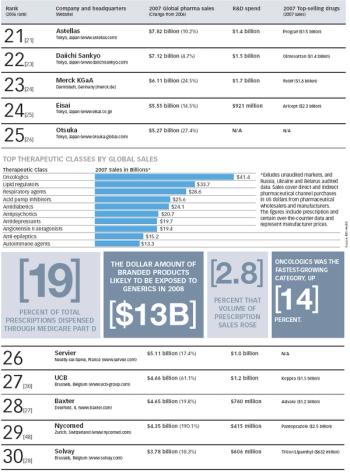
The Pharm Exec 50 report for 2008 accounts for prescription drug sales of more than $510 billion. Interestingly, except for Pfizer, GSK, Sanofi-Aventis (numbers one to three, respectively), and Watson (number 50), every company on the list showed positive growth last year, including some solid double-digit performances.
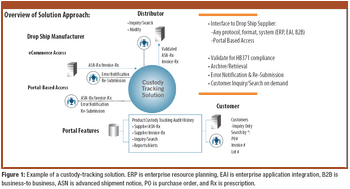
EPedigree, track-and-trace technologies, and other tools for optimizing supply-chain management are of increasing importance to the pharmaceutical industry. The author examines the current regulatory and legislative framework for ePedigree for finished drug products as well as proposals to require electronic statements for pharmaceutical ingredients.
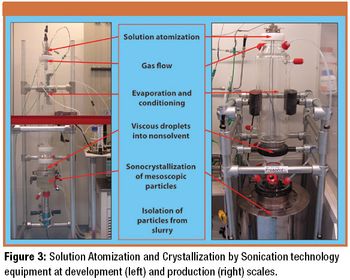
The authors discuss advanced sonocrystallization particle-engineering techniques for manufacturing mesoscopic particles.

The pharmaceutical industry is facing the perfect storm. Increasing healthcare costs, a changing regulatory environment and vigorous global competition coupled with the increasing complexity of small molecule and biotech drugs contributing to expensive discovery processes and clinical trials, as well as the resultant manufacturing challenges, all pose major threats to the industry.

Recombinant microbial whole-cell biocatalysis is a valuable approach for producing enantiomerically pure intermediates. The authors examine several groups of enzymes using this approach: dehydrogenases, hydantoinases, and acylases.
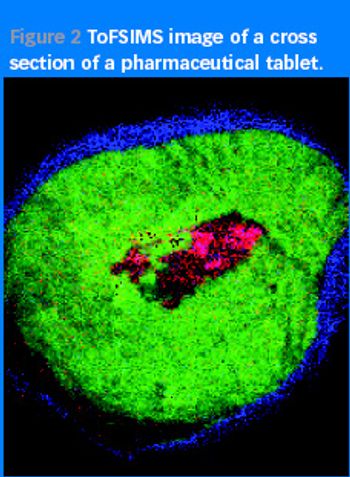
As counterfeiters become more cunning and technologically advanced, spotting their handiwork is increasingly difficult. Can surface analysis techniques be used to outwit them?

The authors describe the critical aspects of an ideal fermentation services provider.

Pharmaceuticals used to be such a simple business when everyone knew their place.

The US Food and Drug Administration will hold a public hearing Oct. 2, 2008, to obtain input regarding over-the-counter (OTC) cough and cold drugs marketed for pediatric use.

The Healthcare Institute of New Jersey (HINJ) released a new research study showing that New Jersey's life-science industry experienced stable growth in 2007.