
Gerresheimer started producing its Gx RTF ClearJect cyclo-olefin copolymer syringes in Germany and will exhibit at CPhI WorldWide 2018.

Gerresheimer started producing its Gx RTF ClearJect cyclo-olefin copolymer syringes in Germany and will exhibit at CPhI WorldWide 2018.

The new features on the purifier deliver additional sizes and sterilization/sanitization compatibility.

The collaboration will focus on developing manufacturing solutions for biosimilars.

ICS distribution center will serve manufacturers of specialty medications, biosimilars, and cell and gene therapies.

Bio-Rad introduces CHT Ceramic Hydroxyapatite XT media and Nuvia HP-Q resin resin for process protein purification.

New products were developed as next-generation process intensification technologies, MilliporeSigma reports.

TruTag Technologies, a provider of product identity solutions, has added mobile-phone authentication of solid oral-dosage form tablets to its product offerings.

Lonza’s new PyroTec Pro Robotic Solution provides a fully automated workflow for endotoxin detection.

The pharmaceutical, clinical, and bioanalytical contract solutions provider has implemented advanced techniques for the collection and use of peripheral blood mononuclear cells (PBMCs) for early-phase clinical trials at its Clinical Pharmacology Unit in Antwerp, Belgium.

The company is increasing manufacturing capacity at its Copenhagen, Denmark facility with the addition of six new bioreactors.

A collaboration integrates Sartorius Stedim Biotech’s BIOSTAT STR bioreactors and Repligen’s XCell ATF cell-retention control technology to create simplified, scalable equipment for intensified cell culture.

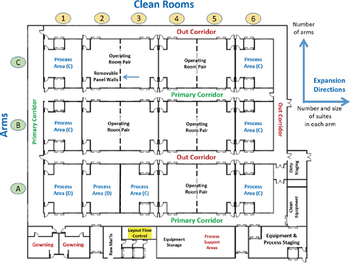
A multi-purpose biopharmaceutical manufacturing facility using a matrix of multi-functional cleanrooms can be adapted to efficiently meet the capacity challenges of both supplying clinical trials and launching products.

The European Commission’s proposed amendment on SPC waivers has sparked opposing views from drug originators and producers of generic drugs and biosimilars.

Watson-Marlow Fluid Technology Group added a new actuator suitable for applications where reduced weight is a concern.

Ross, Charles & Son’s new mixing and discharging turnkey system is capable of extruding finished product into strands.

The SciLog SciPure FD System from Parker Bioscience is an automated single-use system for the filtration and dispensing of products into either bottles or bags.

Sharing know-how can help resolve common bio/pharma technical challenges.

Increasingly complex trial protocols have added to IMP manufacturing challenges.

As counterfeiting, API manufacturing issues, and illegal diversion increase vulnerability, could dispensers and even patients play a greater role in securing the pharma supply chain?

This article focuses on applying new and traditional techniques to design a cleaning process, ensure the surfaces are clean, and develop rinse solution analysis to continuously monitor cleaning performance.

Single-use technologies, modular systems, and robots are on the rise.
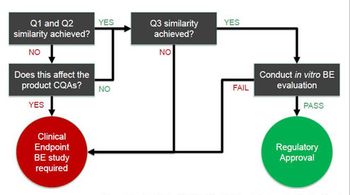
This article examines IVBE testing requirements for topical creams and explores some of the analytical techniques necessary.
Commonly referred to as the future of advanced pharmaceutical manufacturing, continuous manufacturing has gained major traction in the past 10 years to enable significant improvements in efficiency, safety, cost, and speed to market.

Automated robotic arms manipulate nested trays and containers inside closed aseptic filling systems.