
Pre-clinical immunization studies of iBio’s SARS-CoV-2 VLP candidates are being performed by Texas A&M.

Pre-clinical immunization studies of iBio’s SARS-CoV-2 VLP candidates are being performed by Texas A&M.

AzarGen’s biosimilar, made in iBio’s plant-based system, will be compared to the original molecule in pre-clinical studies.

Belgian-based bioprocessing provider, Univercells, has launched a CDMO, Exothera, that will support developers of cell and gene therapies through process development and production of viral vectors.

Understanding of scale-up parameters and use of process analytical technology are important to meet demand for larger batch sizes.

As compounds become more complex in nature and biological ingredients are more widely used, stability testing approaches must follow suit and provide flexibility for developers.

Assays can provide a useful tool in determining the potential toxicity of drugs throughout the development cycle.

Outsourcing stability testing to full-service providers can offer comprehensive benefits to bio/pharma companies.

The huge potential of biopharma is presenting an important epoch for outsourcing partners that can support the development and manufacture of biologics in an efficient way.

Can investing in early formulation studies drive a new therapy successfully across the commercialization finish line?

Eurofins DiscoverX partners with VelaLabs to enable VelaLabs to perform highly reproducible potency lot release assays under GLP/GMP conditions.

Under the agreement, ERS Genomics will license its gene-editing technology to Aelian Biotechnoloy to support its commercial functional genomic screening platform.

Inhalation drug delivery company Vectura announces new organizational structure to drive innovation, customer focus, and growth.

Quick approval pathways challenge teams to balance compliance with the need for speed.

Contract partners must help innovators, especially smaller and virtual companies, consider manufacturability as early as possible in development. This requires focusing on technical and operational performance, as well as cost.

Catalent builds on its investment in cell and gene therapy development and manufacturing with the acquisition of MaSTherCell Global.

In replacing the retiring Paul Hegwood, Ricci Whitlow will oversee global clinical trial operations.

Careful design, planning, and record keeping are needed for cleaning and changeover in multiproduct pharmaceutical facilities.
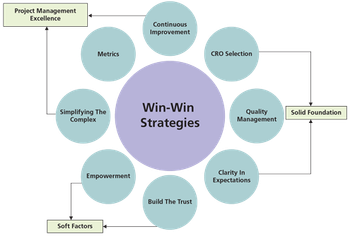
How to adopt win-win strategies and understand quality agreements for complying with cGMP when building strategic relationships with pharmaceutical contract research organizations.

CDMOs must consider challenges associated with the complexity of contract pharmaceutical manufacturing when approaching digitalization projects.
As cell and gene therapies become more prominent, industry is seeking the expertise and capabilities of outsourcing partners to ensure success.

The need for manufacturing speed inspires contract manufacturers to explore advanced processing technologies.

CDMOs and CMOs will continue to invest in biopharmaceutical services and facilities as the bio/pharmaceutical industry looks to biosimilars and personalized medicine.

Drug development contract services company PPD announces initial public offering.

FDA revised the guidance after industry feedback and to clarify CGMP requirements for outsourcing facility operations.

Karen Flynn rejoins Catalent as president of biologics operations; regional presidents named for US and Europe.