
Cambrex reports that the acquisition by the investment group will facilitate ongoing growth.

Cambrex reports that the acquisition by the investment group will facilitate ongoing growth.

Catalent and Ethicann Pharmaceuticals have announced a partnership aimed at developing a new combination pharmaceutical-grade CBD and THC product to treat MS spasticity using Catalent’s orally disintegrating tablet technology.

Spray drying is a versatile and rapid technique that can provide companies with a suitable and scalable option to improve the solubility and bioavailability of drug products.

As regulatory bodies extend the oversight of E&L testing, companies working with drug products need to make provisions on how to best comply with the evolving expectations.

The companies announced a commercial supply agreement following FDA’s accelerated approval.

New training facilities, laboratories, packaging, gene therapy manufacturing, and biologics manufacturing highlight Thermo Fisher Scientific expansions.

Lubrizol Life Science Health adopts new name and opens commercial manufacturing facility.

Fujifilm Diosynth Biotechnologies will add a new building, including gene therapy laboratories, to its facility in College Station, TX.

Unforeseen challenges can be avoided in technology transfer by evaluating the variety of processes involved.

Contract manufacturers are making strategic partnerships and expanding services in the last quarter of 2019.

A complete understanding of primary packaging physicochemical properties is necessary in the formulation and development of biologics.

Lonza, through its Ibex Solutions, will now cover preclinical and clinical development and manufacturing for a significant portion of Genmab’s pipeline.

During the first day of CPhI Worldwide in Frankfurt, Germany, a panel of experts will be discussing the evolution of CDMOs in the shifting therapeutic landscape.

The new services provide rapid production of antibody drug conjugates (ADCs) for best candidate selection.
Limited guidance and numerous challenges create confusion about the scope and timing of stability testing for drugs in development.
As the development pipeline becomes more saturated with complex molecules and patient experience becomes more important, developers are looking to outsourcing partners to provide more specialized expertise and solutions.

Establishing OEL data and ensuring appropriate engineering controls are crucial aspects of safe handling.
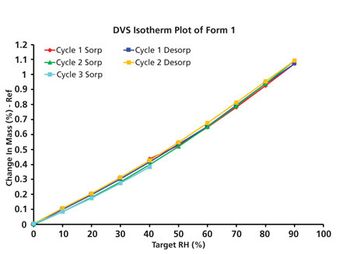
An understanding-during early development-of the solid form landscape of an API can enhance product quality and manufacturing processes.
New therapies, new technologies, global supply chain challenges, and political pressures draw pharma professionals to major industry event.

Bora Pharmaceuticals has announced it has achieved success in the latest FDA general inspection of its Zhunan facility with zero 483 observations.

FDA issued a statement about the importance of reporting adverse events resulting from the use of compounded drugs.

The new antibody, Citryll’s CIT-013, could offer new treatment options for various human diseases including lupus, vasculitis, pulmonary fibrosis, and organ damage due to sepsis.

The editors welcome technical article contributions from the bio/pharma industry.

CDMOs are adding facilities and services to their portfolios in anticipation of the biologics industry’s continued growth.

Alvotech and Prestige Biopharma, have announced the formation of a new contract manufacturing partnership for the commercial production of a biosimilar.