
Think ahead to production requirements when planning strategies in early development of gene and cell therapies.

Think ahead to production requirements when planning strategies in early development of gene and cell therapies.

The companies aim to assess automated CAR-T cell therapy manufacturing at the point-of-care and develop technologies to facilitate patient access to immunotherapies.

The Pharma Services business of Thermo Fisher Scientific will invest $150 Million at three facilities.

Alcami Biologics formed to meet market demands for biological drug development services.

Catalent announces investment for its Zydis ODT technology, offering increased drug load and taste-masking capabilities.

The acquisition of the site in Copenhagen, Denmark, will significantly expand Fujifilm’s capacity and capabilities.

Process optimization improves process mass intensity, reduces environmental impact, and improves cycle time for bio/pharmaceutical processes.

Swedish based company, SenzaGen, has signed a license agreement with contract research organization (CRO) MB Research Labs.
Determination of sodium chloride level is critical for assessment of purity of yeast extracts. This case study demonstrates the validation of an ion chromatography method as a suitable analytical approach.

HPLC coupled with charged aerosol detection is a suitable analytical technique to quantitate and characterize polysorbate-80 in therapeutic products; allowing quality assessment of raw material, content confirmation during manufacturing, and monitoring product stability.

A monoplant may offer greater supply security and flexibility for specialist medicines.

Bio/pharma companies facing new challenges in light of the increasing HPAPI market may benefit from outsourcing.
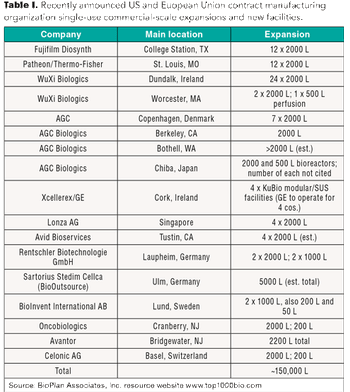
Single-use systems can be a cost savings for CMOs, and these savings can be passed on to clients and, ultimately, to patients.

GHO Capital, a European specialist investor in healthcare, announced its acquisition of Sterling Pharma Solutions, which specializes in complex and difficult-to-manufacture APIs.

Recipharm has received ISO 45001 certification for its Wasserberg facility in Germany as a result of the facility’s program to enhance sustainability and safety.

Investigation of peak purity failure during HPLC method validation led to discovery of a co-eluting impurity under the main peak. Spectral analysis, including three-dimensional modelling, was used to characterize the peak, leading to the development of a new HPLC method for analysing impurity content.

The acquisition would strengthen Charles River’s contract research capabilities.

Catalent adds position of president and chief operating officer to lead sales and quality efforts.

In a partnership with Thermo Fisher Scientific Pharma Services, Pacira added a manufacturing suite in Swindon, UK, that doubles the company’s capacity by mirroring the company’s facility in San Diego, CA.

Nine award winners from the 2019 edition of Pharmapack Europe, covering innovations across drug delivery, packaging, and materials and components, have been announced by the event organizers.

PCI Pharma Services expanded capacity of its commercial packaging site in Illinois.

Radha Iyer, vice-president and head of Quality and Scientific Affairs, Global Developed Markets, Dr. Reddy’s Laboratories, discusses new initiatives at the company.
Keeping abreast of the latest industry trends and retaining flexibility are key to maintaining a strong market position.

Company launches new services dedicated to emerging biotech and biopharma companies.

Novel analytical methods may help biologics manufacturers respond to stricter regulations on particulate matter.